Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Site-Specific Incorporation of 7-Fluoro-L-tryptophan into Proteins by Genetic Encoding to Monitor Ligand Binding by 19F NMR Spectroscopy
ACS Sensors ( IF 8.2 ) Pub Date : 2022-01-10 , DOI: 10.1021/acssensors.1c02467 Haocheng Qianzhu 1 , Elwy H Abdelkader 2 , Iresha D Herath 2 , Gottfried Otting 2 , Thomas Huber 1
ACS Sensors ( IF 8.2 ) Pub Date : 2022-01-10 , DOI: 10.1021/acssensors.1c02467 Haocheng Qianzhu 1 , Elwy H Abdelkader 2 , Iresha D Herath 2 , Gottfried Otting 2 , Thomas Huber 1
Affiliation
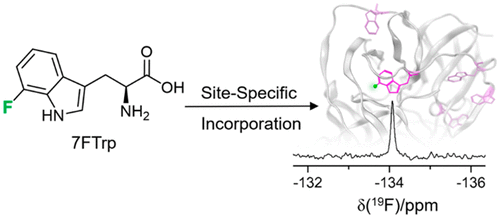
|
A mutant aminoacyl-tRNA synthetase identified by a library selection system affords site-specific incorporation of 7-fluoro-L-tryptophan in response to an amber stop codon. The enzyme allows the production of proteins with a single hydrogen atom replaced by a fluorine atom as a sensitive nuclear magnetic resonance (NMR) probe. The substitution of a single hydrogen atom by another element that is as closely similar in size and hydrophobicity as possible minimizes possible perturbations in the structure, stability, and solubility of the protein. The fluorine atom enables site-selective monitoring of the protein response to ligand binding by 19F NMR spectroscopy, as demonstrated with the Zika virus NS2B-NS3 protease.
中文翻译:

通过基因编码将 7-氟-L-色氨酸位点特异性掺入蛋白质中以通过 19F NMR 光谱监测配体结合
由文库选择系统鉴定的突变氨酰-tRNA 合成酶响应琥珀终止密码子提供了 7-氟-L-色氨酸的位点特异性掺入。该酶允许生产具有被氟原子取代的单个氢原子的蛋白质,作为敏感的核磁共振 (NMR) 探针。将单个氢原子替换为大小和疏水性尽可能相似的另一种元素可以最大限度地减少蛋白质结构、稳定性和溶解度的可能扰动。正如寨卡病毒 NS2B-NS3 蛋白酶所证明的,氟原子能够通过19 F NMR 光谱对配体结合的蛋白质反应进行位点选择性监测。
更新日期:2022-01-28
中文翻译:

通过基因编码将 7-氟-L-色氨酸位点特异性掺入蛋白质中以通过 19F NMR 光谱监测配体结合
由文库选择系统鉴定的突变氨酰-tRNA 合成酶响应琥珀终止密码子提供了 7-氟-L-色氨酸的位点特异性掺入。该酶允许生产具有被氟原子取代的单个氢原子的蛋白质,作为敏感的核磁共振 (NMR) 探针。将单个氢原子替换为大小和疏水性尽可能相似的另一种元素可以最大限度地减少蛋白质结构、稳定性和溶解度的可能扰动。正如寨卡病毒 NS2B-NS3 蛋白酶所证明的,氟原子能够通过19 F NMR 光谱对配体结合的蛋白质反应进行位点选择性监测。







































 京公网安备 11010802027423号
京公网安备 11010802027423号