当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt-Catalyzed Enantioselective C–H Arylation of Indoles
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-01-10 , DOI: 10.1021/jacs.1c09889 Nicolas Jacob 1 , Yassir Zaid 1 , João C A Oliveira 2 , Lutz Ackermann 2, 3 , Joanna Wencel-Delord 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-01-10 , DOI: 10.1021/jacs.1c09889 Nicolas Jacob 1 , Yassir Zaid 1 , João C A Oliveira 2 , Lutz Ackermann 2, 3 , Joanna Wencel-Delord 1
Affiliation
|
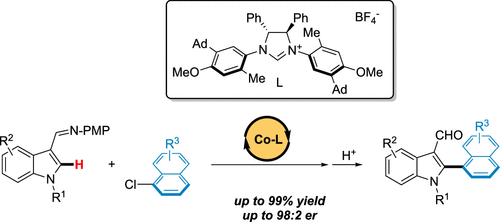
Atropoisomeric (hetero)biaryls are scaffolds with increasing importance in the pharmaceutical and agrochemical industries. Although it is the most obvious disconnection to construct such compounds, the direct enantioselective C–H arylation through the concomitant induction of the chiral information remains extremely challenging and uncommon. Herein, the unprecedented earth-abundant 3d-metal-catalyzed atroposelective direct arylation is reported, furnishing rare atropoisomeric C2-arylated indoles. Kinetic studies and DFT computation revealed an uncommon mechanism for this asymmetric transformation, with the oxidative addition being the rate- and enantio-determining step. Excellent stereoselectivities were reached (up to 96% ee), while using an unusual N-heterocyclic carbene ligand bearing an essential remote substituent. Attractive dispersion interactions along with positive C–H---π interactions exerted by the ligand were identified as key factors to guarantee the excellent enantioselection.
中文翻译:

钴催化吲哚的对映选择性 C-H 芳基化
阻转异构(杂)联芳基是在制药和农业化学工业中越来越重要的支架。尽管构建此类化合物是最明显的分离,但通过同时诱导手性信息的直接对映选择性 C-H 芳基化仍然极具挑战性且不常见。在这里,报道了前所未有的地球丰富的 3d 金属催化的阻转选择性直接芳基化,提供了罕见的阻转异构 C2 芳基化吲哚。动力学研究和 DFT 计算揭示了这种不对称转变的不常见机制,氧化加成是决定速率和对映体的步骤。达到了出色的立体选择性(高达 96% ee),同时使用不寻常的N-杂环卡宾配体带有一个必要的远程取代基。有吸引力的色散相互作用以及配体施加的正 C-H---π 相互作用被确定为保证优异对映体选择的关键因素。
更新日期:2022-01-19
中文翻译:

钴催化吲哚的对映选择性 C-H 芳基化
阻转异构(杂)联芳基是在制药和农业化学工业中越来越重要的支架。尽管构建此类化合物是最明显的分离,但通过同时诱导手性信息的直接对映选择性 C-H 芳基化仍然极具挑战性且不常见。在这里,报道了前所未有的地球丰富的 3d 金属催化的阻转选择性直接芳基化,提供了罕见的阻转异构 C2 芳基化吲哚。动力学研究和 DFT 计算揭示了这种不对称转变的不常见机制,氧化加成是决定速率和对映体的步骤。达到了出色的立体选择性(高达 96% ee),同时使用不寻常的N-杂环卡宾配体带有一个必要的远程取代基。有吸引力的色散相互作用以及配体施加的正 C-H---π 相互作用被确定为保证优异对映体选择的关键因素。






























 京公网安备 11010802027423号
京公网安备 11010802027423号