当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total Syntheses of Isodomoic Acids G and H
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-10-14 , DOI: 10.1021/ja9063475 Scott E Denmark 1 , Jack Hung-Chang Liu , Joseck M Muhuhi
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-10-14 , DOI: 10.1021/ja9063475 Scott E Denmark 1 , Jack Hung-Chang Liu , Joseck M Muhuhi
Affiliation
|
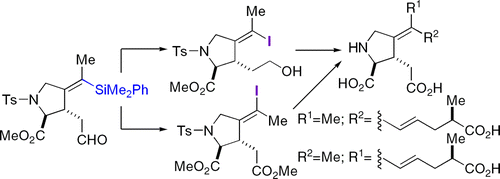
The total syntheses of marine natural products belonging to the kainoid family, isodomoic acids G and H, are described. The strategic connection involves a sequential silylcarbocyclization/silicon-based cross-coupling process. These total syntheses were achieved efficiently via a 12- and a 13-step, longest-linear sequence, respectively. The key transformations include a diastereoselective rhodium-catalyzed carbonylative silylcarbocyclization reaction of an (l)-vinylglycine-derived 1,6-enyne, a desilylative iodination reaction, as well as an alkenyl-alkenyl silicon-based cross-coupling reaction. The mechanistic insight garnered during the investigation of the iododesilylation reaction enabled stereocontrolled introduction of the iodine with either inversion or retention of double bond configuration. The invertive desilylative iodination leads to the total synthesis of isodomoic acid H, while its congener, isodomoic acid G, was obtained via a retentive iododesilylation.
中文翻译:

异糖酸 G 和 H 的全合成
描述了属于 kainoid 家族、isodomoic 酸 G 和 H 的海洋天然产物的全合成。战略联系涉及顺序的甲硅烷基碳环化/基于硅的交叉偶联过程。这些全合成分别通过 12 步和 13 步最长线性序列有效实现。关键转化包括(l)-乙烯基甘氨酸衍生的 1,6-烯炔的非对映选择性铑催化羰基化甲硅烷基碳环化反应、脱甲硅烷基碘化反应以及基于烯基-烯基硅的交叉偶联反应。在研究碘脱甲硅烷基化反应过程中获得的机械洞察力使碘的立体控制引入与双键构型的反转或保留成为可能。
更新日期:2009-10-14
中文翻译:

异糖酸 G 和 H 的全合成
描述了属于 kainoid 家族、isodomoic 酸 G 和 H 的海洋天然产物的全合成。战略联系涉及顺序的甲硅烷基碳环化/基于硅的交叉偶联过程。这些全合成分别通过 12 步和 13 步最长线性序列有效实现。关键转化包括(l)-乙烯基甘氨酸衍生的 1,6-烯炔的非对映选择性铑催化羰基化甲硅烷基碳环化反应、脱甲硅烷基碘化反应以及基于烯基-烯基硅的交叉偶联反应。在研究碘脱甲硅烷基化反应过程中获得的机械洞察力使碘的立体控制引入与双键构型的反转或保留成为可能。












































 京公网安备 11010802027423号
京公网安备 11010802027423号