当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Oxygen Redox Activity by High-Valent Fe and Co3+ Sites in the Perovskite LaNi1–xFe0.5xCo0.5xO3
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-01-07 , DOI: 10.1021/acsaem.1c02871 Anjaiah Sheelam, Sakthipriya Balu, Adil Muneeb, Khasim Saheb Bayikadi, Dhenadhayalan Namasivayam, Erakulan E. Siddharthan, Arif I. Inamdar, Ranjit Thapa, Ming-Hsi Chiang, Song-Jeng Isaac Huang, Raman Sankar
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-01-07 , DOI: 10.1021/acsaem.1c02871 Anjaiah Sheelam, Sakthipriya Balu, Adil Muneeb, Khasim Saheb Bayikadi, Dhenadhayalan Namasivayam, Erakulan E. Siddharthan, Arif I. Inamdar, Ranjit Thapa, Ming-Hsi Chiang, Song-Jeng Isaac Huang, Raman Sankar
|
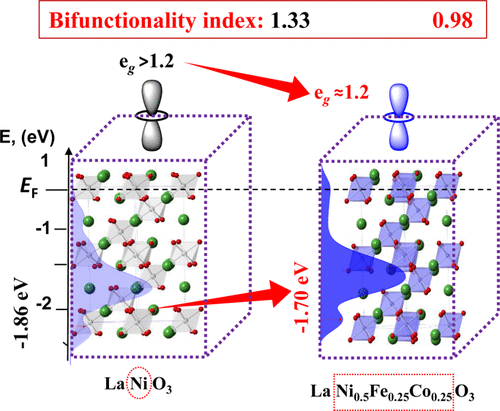
Tuning the electronic structure of perovskite oxides via aliovalent substitution is a promising strategy to attain inexpensive and efficient electrocatalysts for energy conversion and storage devices. Herein, following the d-band center positions and using a simple sol–gel method followed by a pyrolysis step, LaNi1–xCo0.5xFe0.5xO3 (LNFCO-x; x = 0.0, 0.4, 0.5, and 0.6) electrocatalysts are designed and synthesized for oxygen redox reactions in 1 M KOH. Among them, LNFCO-0.5 has exhibited the lowest overpotential and the highest charge transfer kinetics in oxygen redox reactions. Overall, a 90 mV lower overpotential was observed in oxygen redox activity of LNFCO-0.5 compared to that of pristine LaNiO3. The mass activity of LNFCO-0.5 in the oxygen reduction reaction (at 0.7 V vs RHE) and oxygen evolution reaction (1.60 V vs RHE) was calculated to be 2.5 and 2.13 times higher than that of LaNiO3, respectively. The bifunctionality index (potential difference between the oxygen evolution at a current density of 10 mA cm–2 and the oxygen reduction at a current density of −1 mA cm–2) of LNFCO-0.5 was found to be 0.98. The substitution of Fe and Co for the Ni-site shifted the d-band center close to the Fermi level, which can increase the binding strength of the *OH intermediate in the rate-determining step. Also, the surface was enriched with Fe3+Δ, Co3+, and partially oxidized Ni3+ states, which is susceptible to tune the eg-orbital filling for superior oxygen redox activity.
中文翻译:

通过钙钛矿 LaNi1–xFe0.5xCo0.5xO3 中的高价 Fe 和 Co3+ 位点提高氧的氧化还原活性
通过异价取代调整钙钛矿氧化物的电子结构是获得用于能量转换和存储设备的廉价且高效的电催化剂的有前景的策略。在此,遵循 d 带中心位置并使用简单的溶胶-凝胶法,然后进行热解步骤,LaNi 1– x Co 0.5 x Fe 0.5 x O 3 (LNFCO- x ; x= 0.0、0.4、0.5 和 0.6) 设计和合成电催化剂用于 1 M KOH 中的氧氧化还原反应。其中,LNFCO-0.5在氧氧化还原反应中表现出最低的过电位和最高的电荷转移动力学。总体而言,与原始 LaNiO 3相比,LNFCO-0.5 的氧氧化还原活性观察到低 90 mV 的过电位。LNFCO-0.5 在氧还原反应(相对于 RHE 为 0.7 V)和析氧反应(相对于 RHE 为 1.60 V)中的质量活度分别是 LaNiO 3的 2.5 倍和 2.13 倍。双功能指数(电流密度为 10 mA cm –2时的氧释放与电流密度为 -1 mA cm –2时的氧还原之间的电位差) 的 LNFCO-0.5 被发现为 0.98。Fe 和 Co 取代 Ni 位点使 d 带中心移动到接近费米能级的位置,这可以增加*OH 中间体在速率决定步骤中的结合强度。此外,表面富含Fe 3+Δ、Co 3+和部分氧化的Ni 3+态,这易于调节e g轨道填充以获得优异的氧氧化还原活性。
更新日期:2022-01-24
中文翻译:

通过钙钛矿 LaNi1–xFe0.5xCo0.5xO3 中的高价 Fe 和 Co3+ 位点提高氧的氧化还原活性
通过异价取代调整钙钛矿氧化物的电子结构是获得用于能量转换和存储设备的廉价且高效的电催化剂的有前景的策略。在此,遵循 d 带中心位置并使用简单的溶胶-凝胶法,然后进行热解步骤,LaNi 1– x Co 0.5 x Fe 0.5 x O 3 (LNFCO- x ; x= 0.0、0.4、0.5 和 0.6) 设计和合成电催化剂用于 1 M KOH 中的氧氧化还原反应。其中,LNFCO-0.5在氧氧化还原反应中表现出最低的过电位和最高的电荷转移动力学。总体而言,与原始 LaNiO 3相比,LNFCO-0.5 的氧氧化还原活性观察到低 90 mV 的过电位。LNFCO-0.5 在氧还原反应(相对于 RHE 为 0.7 V)和析氧反应(相对于 RHE 为 1.60 V)中的质量活度分别是 LaNiO 3的 2.5 倍和 2.13 倍。双功能指数(电流密度为 10 mA cm –2时的氧释放与电流密度为 -1 mA cm –2时的氧还原之间的电位差) 的 LNFCO-0.5 被发现为 0.98。Fe 和 Co 取代 Ni 位点使 d 带中心移动到接近费米能级的位置,这可以增加*OH 中间体在速率决定步骤中的结合强度。此外,表面富含Fe 3+Δ、Co 3+和部分氧化的Ni 3+态,这易于调节e g轨道填充以获得优异的氧氧化还原活性。













































 京公网安备 11010802027423号
京公网安备 11010802027423号