当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
β-Methyltryptamine Provoking the Crucial Role of Strictosidine Synthase Tyr151-OH for Its Stereoselective Pictet−Spengler Reactions to Tryptoline-type Alkaloids
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2022-01-07 , DOI: 10.1021/acschembio.1c00844 Haicheng Liu 1 , Santosh Panjikar 2 , Xiang Sheng 3 , Yushi Futamura 4 , Chenghua Zhang 3, 5 , Nana Shao 1 , Hiroyuki Osada 4 , Hongbin Zou 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2022-01-07 , DOI: 10.1021/acschembio.1c00844 Haicheng Liu 1 , Santosh Panjikar 2 , Xiang Sheng 3 , Yushi Futamura 4 , Chenghua Zhang 3, 5 , Nana Shao 1 , Hiroyuki Osada 4 , Hongbin Zou 1
Affiliation
|
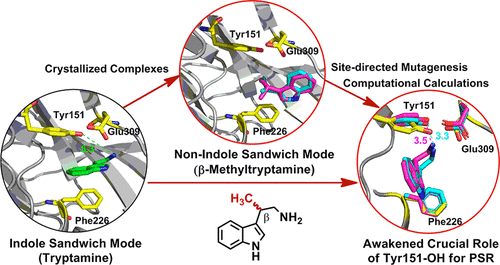
Strictosidine synthase (STR), the gate enzyme for monoterpenoid indole alkaloid biosynthesis, catalyzes the Pictet–Spengler reaction (PSR) of various tryptamine derivatives with secologanin assisted by “indole sandwich” stabilization. Continuous exploration with β-methyltryptamine (IPA) stereoselectively delivered the C6-methylstrictosidines and C6-methylvincosides by enzymatic and nonenzymatic PSR, respectively. Unexpectedly, the first “nonindole sandwich” binding mode was witnessed by the X-ray structures of STR1-ligand complexes. Site-directed mutagenesis revealed the critical cryptic role of the hydroxyl group of Tyr151 in IPA biotransformation. Further computational calculations demonstrated the adjustable IPA position in STR1 upon the binding of secologanin, and Tyr151-OH facilitates the productive PSR binding mode via an advantageous hydrogen-bond network. Further chemo-enzymatic manipulation of C6-methylvincosides successfully resulted in the discovered antimalarial framework (IC50 = 0.92 μM).
中文翻译:

β-甲基色胺激发了马球糖苷合酶 Tyr151-OH 在其对色氨酸型生物碱的立体选择性 Pictet−Spengler 反应中的关键作用
马球糖苷合酶 (STR) 是单萜吲哚生物碱生物合成的门酶,在“吲哚三明治”稳定作用的辅助下,催化各种色胺衍生物与赛洛甘宁的 Pictet-Spengler 反应 (PSR)。使用 β-甲基色胺 (IPA) 进行持续探索,分别通过酶促和非酶促 PSR 立体选择性地递送 C6-甲基胡桃素和 C6-甲基长春糖苷。出乎意料的是,STR1-配体复合物的X射线结构见证了第一个“非吲哚夹心”结合模式。定点诱变揭示了 Tyr151 羟基在 IPA 生物转化中的关键神秘作用。进一步的计算表明,与 secologanin 结合后,STR1 中的 IPA 位置可调节,而 Tyr151-OH 通过有利的氢键网络促进高效的 PSR 结合模式。 C6-甲基长春糖苷的进一步化学酶操作成功地产生了所发现的抗疟框架(IC 50 = 0.92 μM)。
更新日期:2022-01-21
中文翻译:

β-甲基色胺激发了马球糖苷合酶 Tyr151-OH 在其对色氨酸型生物碱的立体选择性 Pictet−Spengler 反应中的关键作用
马球糖苷合酶 (STR) 是单萜吲哚生物碱生物合成的门酶,在“吲哚三明治”稳定作用的辅助下,催化各种色胺衍生物与赛洛甘宁的 Pictet-Spengler 反应 (PSR)。使用 β-甲基色胺 (IPA) 进行持续探索,分别通过酶促和非酶促 PSR 立体选择性地递送 C6-甲基胡桃素和 C6-甲基长春糖苷。出乎意料的是,STR1-配体复合物的X射线结构见证了第一个“非吲哚夹心”结合模式。定点诱变揭示了 Tyr151 羟基在 IPA 生物转化中的关键神秘作用。进一步的计算表明,与 secologanin 结合后,STR1 中的 IPA 位置可调节,而 Tyr151-OH 通过有利的氢键网络促进高效的 PSR 结合模式。 C6-甲基长春糖苷的进一步化学酶操作成功地产生了所发现的抗疟框架(IC 50 = 0.92 μM)。































 京公网安备 11010802027423号
京公网安备 11010802027423号