当前位置:
X-MOL 学术
›
Chem. Heterocycl. Comp.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of methyl(ethyl) pyrazolo[4,3-b]pyridine-6-carboxylates and their conversion to tert-butyl 4,5,6,7-tetrahydropyrazolo-[4,3-b]pyridine-6-carboxylates
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2022-01-06 , DOI: 10.1007/s10593-021-03032-z Georgiy G. Yakovenko 1, 2 , Eduard B. Rusanov 2 , Mykhailo V. Vovk 2 , Lesya N. Saliyeva 3 , Il’ya S. Donchak 4
中文翻译:

甲基(乙基)吡唑并[4,3-b]吡啶-6-羧酸酯的合成及其向4,5,6,7-四氢吡唑并-[4,3-b]吡啶-6-羧酸叔丁酯的转化
更新日期:2022-01-06
Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2022-01-06 , DOI: 10.1007/s10593-021-03032-z Georgiy G. Yakovenko 1, 2 , Eduard B. Rusanov 2 , Mykhailo V. Vovk 2 , Lesya N. Saliyeva 3 , Il’ya S. Donchak 4
Affiliation
|
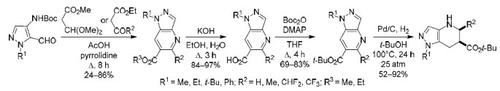
N-Boc-4-aminopyrazole-5-carbaldehydes react with methyl 3,3-dimethoxypropanoate or β-keto esters in acetic acid under reflux to form methyl(ethyl) pyrazolo[4,3-b]pyridine-6-carboxylates, which were converted to the corresponding tert-butyl carboxylates via intermediate carboxylic acids. Their subsequent hydrogenation on a 10% Pd/C catalyst at 100°C and 25 atm afforded tert-butyl 4,5,6,7-tetrahydropyrazolo[4,3-b]pyridine-6-carboxylates.
中文翻译:

甲基(乙基)吡唑并[4,3-b]吡啶-6-羧酸酯的合成及其向4,5,6,7-四氢吡唑并-[4,3-b]吡啶-6-羧酸叔丁酯的转化
N -Boc-4-氨基吡唑-5-甲醛与3,3-二甲氧基丙酸甲酯或β-酮酯在乙酸中回流反应生成吡唑并[4,3 - b ]吡啶-6-羧酸甲酯(通过中间体羧酸转化为相应的羧酸叔丁酯。它们随后在 10% Pd/C 催化剂上在 100°C 和 25 个大气压下氢化,得到4,5,6,7-四氢吡唑并[4,3 - b ]吡啶-6-羧酸叔丁酯。








































 京公网安备 11010802027423号
京公网安备 11010802027423号