当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
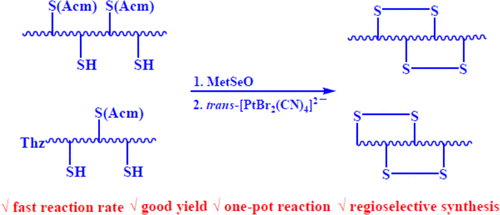
Deprotection of S-Acetamidomethyl and 1,3-Thiazolidine-4-Carbonyl Protecting Groups from Cysteine Side Chains in Peptides by trans-[PtX2(CN)4]2–: One-Pot Regioselective Synthesis of Disulfide Bonds
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-01-05 , DOI: 10.1021/acs.joc.1c02793 Dongying Ma 1 , Jingjing Sun 1 , Shigang Shen 1 , Hua Chen 1 , Wenzhi Xu 1 , Yafang Wang 1 , Changying Song 1 , Tiesheng Shi 2 , Shuying Huo 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-01-05 , DOI: 10.1021/acs.joc.1c02793 Dongying Ma 1 , Jingjing Sun 1 , Shigang Shen 1 , Hua Chen 1 , Wenzhi Xu 1 , Yafang Wang 1 , Changying Song 1 , Tiesheng Shi 2 , Shuying Huo 1
Affiliation
|
In this study, we developed an efficient approach for disulfide bond formation in peptides utilizing the Pt(IV) complex trans-[PtBr2(CN)4]2– to mediate Acm and Thz deprotections. [PtBr2(CN)4]2– can oxidatively deprotect two Acm groups or deprotect one Thz group and one Acm group to directly form an intramolecular disulfide bond in peptides. Several disulfide-containing peptides with excellent yields were achieved via the deprotection method in an aqueous medium under aerobic conditions. Kinetic studies indicated that the dominant path of the reaction is of first-order in both [Pt(IV)] and [peptide]; moreover, the deprotection rate increased dramatically with the addition of NaBr. A mechanism including a bromide-bridge-mediated electron transfer process was proposed. Apamin, α-conotoxin SI, and the parallel homodimer of oxytocin, all containing two disulfide bonds, were synthesized regioselectively through a one-pot method by the combined use of the above deprotection approach with oxidants l-methionine selenoxide and [PtBr2(CN)4]2–. All of the reactions were completed within 30 min to afford good yields for these peptides.
中文翻译:

反式-[PtX2(CN)4]2– 对肽中半胱氨酸侧链的 S-乙酰氨基甲基和 1,3-噻唑烷-4-羰基保护基团的脱保护:二硫键的一锅区域选择性合成
在这项研究中,我们开发了一种利用 Pt(IV) 复合物反式-[PtBr 2 (CN) 4 ] 2-介导 Acm 和 Thz 去保护的肽中二硫键形成的有效方法。[PtBr 2 (CN) 4 ] 2–可以氧化去保护两个Acm基团或去保护一个Thz基团和一个Acm基团,直接在肽中形成分子内二硫键。在有氧条件下,在水性介质中通过脱保护方法获得了几种具有优异产率的含二硫键肽。动力学研究表明,[Pt(IV)]和[肽]反应的主要途径是一级反应;此外,随着 NaBr 的加入,脱保护率显着提高。提出了一种包括溴化物桥介导的电子转移过程的机制。Apamin、α-芋螺毒素 SI 和催产素的平行同型二聚体均含有两个二硫键,通过一锅法结合使用上述脱保护方法与氧化剂l区域选择性合成-甲硫氨酸硒氧化物和[PtBr 2 (CN) 4 ] 2-。所有反应均在 30 分钟内完成,从而为这些肽提供了良好的收率。
更新日期:2022-01-21
中文翻译:

反式-[PtX2(CN)4]2– 对肽中半胱氨酸侧链的 S-乙酰氨基甲基和 1,3-噻唑烷-4-羰基保护基团的脱保护:二硫键的一锅区域选择性合成
在这项研究中,我们开发了一种利用 Pt(IV) 复合物反式-[PtBr 2 (CN) 4 ] 2-介导 Acm 和 Thz 去保护的肽中二硫键形成的有效方法。[PtBr 2 (CN) 4 ] 2–可以氧化去保护两个Acm基团或去保护一个Thz基团和一个Acm基团,直接在肽中形成分子内二硫键。在有氧条件下,在水性介质中通过脱保护方法获得了几种具有优异产率的含二硫键肽。动力学研究表明,[Pt(IV)]和[肽]反应的主要途径是一级反应;此外,随着 NaBr 的加入,脱保护率显着提高。提出了一种包括溴化物桥介导的电子转移过程的机制。Apamin、α-芋螺毒素 SI 和催产素的平行同型二聚体均含有两个二硫键,通过一锅法结合使用上述脱保护方法与氧化剂l区域选择性合成-甲硫氨酸硒氧化物和[PtBr 2 (CN) 4 ] 2-。所有反应均在 30 分钟内完成,从而为这些肽提供了良好的收率。












































 京公网安备 11010802027423号
京公网安备 11010802027423号