当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of novel (E)-N′-(2,3-dihydro-1H-inden-1-ylidene) benzohydrazides as potent LSD1 inhibitors
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2016-08-30 14:26:52 Yang Zhou, Yan Li, Wen-Jing Wang, Pu Xiang, Xin-Mei Luo, Li Yang, Sheng-Yong Yang, Ying-Lan Zhao
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2016-08-30 14:26:52 Yang Zhou, Yan Li, Wen-Jing Wang, Pu Xiang, Xin-Mei Luo, Li Yang, Sheng-Yong Yang, Ying-Lan Zhao
|
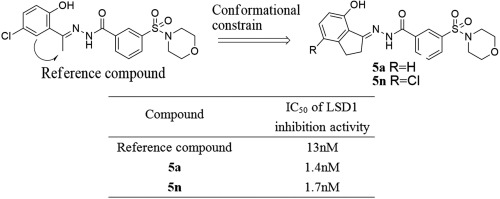
Lysine specific demethylase 1 (LSD1) plays an important role in regulating histone lysine methylation at residues K4 and K9 on histone H3 and is recognized as an attractive therapeutic target in multiple malignancies. In this study, a series of novel (E)-N′-(2,3-dihydro-1H-inden-1-ylidene) benzohydrazides were synthesized and biologically evaluated for their potential LSD1 inhibitory effect. Among them, compounds 5a and 5n showed the most potent LSD1 inhibitory activity with IC50 values of 1.4 and 1.7nM, respectively, which were about 10 times more potent compared with (E)-N-(1-(5-chloro-2-hydroxyphenyl) ethylidene)-3-(morpholinosulf-only) benzohydrazide (J. Med. Chem. 2013, 56, 9496–9508; as reference compound). Compounds 5a and 5n also exhibited marked anti-proliferation activities against cancer cell lines that highly expressed LSD1. These results suggest that these optimized compounds might be served as promising LSD1 inhibitors against cancer, which merit further study.
中文翻译:

新型(E)-N'-(2,3-二氢-1H-茚满-1-亚烷基)苯甲酰肼作为强力LSD1抑制剂的合成及生物学评价
赖氨酸特异性脱甲基酶1(LSD1)在调节组蛋白H3上K4和K9残基处的组蛋白赖氨酸甲基化中起着重要作用,并且被认为是多种恶性肿瘤中有吸引力的治疗靶标。在这项研究中,合成了一系列新颖的(E)-N'-(2,3-二氢-1H-茚满-1-亚烷基)苯并肼,并对它们对LSD1的潜在抑制作用进行了生物学评估。其中,化合物5a和5n对IC 50表现出最强的LSD1抑制活性分别为1.4和1.7nM值,与(E)-N-(1-(5-氯-2-羟基苯基)亚乙基)-3-(仅吗啉代亚砜)苯并酰肼相比,效价高约10倍(J. Med.Chem.2013,56,9496–9508;作为参考化合物)。化合物5a和5n还对高表达LSD1的癌细胞系表现出显着的抗增殖活性。这些结果表明,这些优化的化合物可作为有希望的抗癌LSD1抑制剂,值得进一步研究。
更新日期:2016-08-31
中文翻译:

新型(E)-N'-(2,3-二氢-1H-茚满-1-亚烷基)苯甲酰肼作为强力LSD1抑制剂的合成及生物学评价
赖氨酸特异性脱甲基酶1(LSD1)在调节组蛋白H3上K4和K9残基处的组蛋白赖氨酸甲基化中起着重要作用,并且被认为是多种恶性肿瘤中有吸引力的治疗靶标。在这项研究中,合成了一系列新颖的(E)-N'-(2,3-二氢-1H-茚满-1-亚烷基)苯并肼,并对它们对LSD1的潜在抑制作用进行了生物学评估。其中,化合物5a和5n对IC 50表现出最强的LSD1抑制活性分别为1.4和1.7nM值,与(E)-N-(1-(5-氯-2-羟基苯基)亚乙基)-3-(仅吗啉代亚砜)苯并酰肼相比,效价高约10倍(J. Med.Chem.2013,56,9496–9508;作为参考化合物)。化合物5a和5n还对高表达LSD1的癌细胞系表现出显着的抗增殖活性。这些结果表明,这些优化的化合物可作为有希望的抗癌LSD1抑制剂,值得进一步研究。







































 京公网安备 11010802027423号
京公网安备 11010802027423号