Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-01-03 , DOI: 10.1016/j.jhazmat.2021.128191 Linlu Shen 1 , Zhonglin Chen 1 , Jing Kang 1 , Pengwei Yan 1 , Jimin Shen 1 , Binyuan Wang 1 , Shengxin Zhao 1 , Lanbo Bi 1 , Shuyu Wang 1 , Yizhen Cheng 1
|
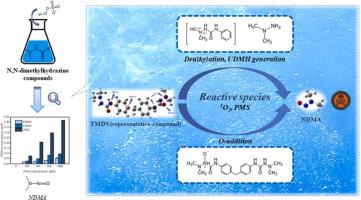
This study found that peroxymonosulfate (PMS) oxidation without activation has the potential to generate a suspected human carcinogen, N-nitrosodimethylamine (NDMA), in water containing N,N-dimethylhydrazine compounds. Considerable amounts of NDMA formed from three compounds by PMS oxidation were observed. 1,1,1′,1′-Tetramethyl-4,4′-(methylene-di-p-phenylene) disemicarbazide (TMDS), which is an industrial antiyellowing agent and light stabilizer, was used as a representative to elucidate the kinetics, transformation products, mechanism and NDMA formation pathways of PMS oxidation. TMDS degradation and NDMA formation involved direct PMS oxidation and singlet oxygen (1O2) oxidation. The oxidation by PMS/1O2 was pH-dependent, which was related to the pH-dependent characteristics of the reactive oxygen species and intermediates. The degradation mechanism of TMDS mainly included the side chain cleavage, dealkylation, and O-addition. NDMA was generated from TMDS mainly via O-addition and 1,1-dimethylhydrazine (UDMH) generation. The cleavage of amide nitrogen in O-addition products and primary amine nitrogen in UDMH are likely the key steps in NDMA generation. The results emphasized that the formation of harmful by-products should be taken into account when assessing the feasibility of PMS oxidation.
中文翻译:

过氧单硫酸盐氧化 N,N-二甲基肼化合物过程中 N-亚硝基二甲胺的形成:动力学、反应物种、机理和影响因素
这项研究发现,未经活化的过氧单硫酸盐 (PMS) 氧化有可能在含有 N,N-二甲基肼化合物的水中产生疑似人类致癌物 N-亚硝基二甲胺 (NDMA)。观察到通过 PMS 氧化由三种化合物形成的大量 NDMA。以工业抗黄变剂和光稳定剂1,1,1',1'-Tetramethyl-4,4'-(methylene-di-p-phenylene) disemicarbazide (TMDS)为代表阐明动力学PMS氧化的转化产物、机理和NDMA形成途径。TMDS 降解和 NDMA 形成涉及直接 PMS 氧化和单线态氧 ( 1 O 2 ) 氧化。PMS/ 1 O 2氧化是pH依赖性的,这与活性氧和中间体的pH依赖性特征有关。TMDS的降解机理主要包括侧链裂解、脱烷基化和O-加成。NDMA 主要通过 O-加成和 1,1-二甲基肼 (UDMH) 生成从 TMDS 生成。O-加成产物中酰胺氮的裂解和 UDMH 中伯胺氮的裂解可能是 NDMA 生成的关键步骤。结果强调,在评估 PMS 氧化的可行性时,应考虑有害副产物的形成。















































 京公网安备 11010802027423号
京公网安备 11010802027423号