Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-12-31 , DOI: 10.1016/j.cej.2021.134322 Yongyang Zhu 1, 2 , Shaoyang Shen 1 , Liuzhang Ouyang 1, 3 , Jiangwen Liu 1 , Hui Wang 1 , Zhenguo Huang 4 , Min Zhu 1
|
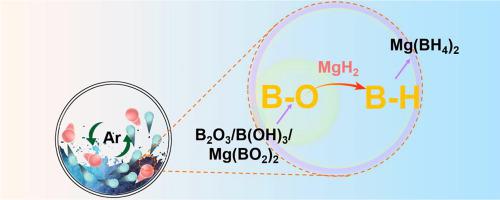
Magnesium borohydride (Mg(BH4)2) is widely regarded as a promising hydrogen storage material due to its high capacity; however, it is still challenging to synthesize Mg(BH4)2 with low cost. Traditionally, Mg(BH4)2 has been mainly produced using other borohydride as the starting materials via exchange reactions. Herein, we report an economical method to synthesize Mg(BH4)2 by converting B-O bonds in widely available borates or boric acid to B-H. The borates or boric acid is ball-milled with MgH2 under ambient conditions to form Mg(BH4)2 with high yield (>80%). Mg(BH4)2 was also successfully generated by reacting low-cost Mg with boric acid. Compared with previous approaches, this method avoids expensive boron sources such as LiBH4, NaBH4, and B2H6, and does not require high pressure H2 gas and high temperatures, and therefore significantly reduces costs. This method could be an alternative to the current Mg(BH4)2 synthesis processes.
中文翻译:

通过BO到BH键转换有效合成硼氢化镁
硼氢化镁(Mg(BH 4 ) 2 )因其高容量而被广泛认为是一种很有前途的储氢材料。然而,以低成本合成Mg(BH 4 ) 2仍然具有挑战性。传统上,Mg(BH 4 ) 2主要使用其他硼氢化物作为原料通过交换反应生产。在此,我们报告了一种通过将广泛可用的硼酸盐或硼酸中的 BO 键转化为BH 来合成 Mg(BH 4 ) 2的经济方法。硼酸盐或硼酸在环境条件下与 MgH 2球磨形成 Mg(BH 4 ) 2收率高(>80%)。Mg(BH 4 ) 2也可以通过低成本的镁与硼酸反应成功生成。与之前的方法相比,该方法避免了LiBH 4、NaBH 4和B 2 H 6等昂贵的硼源,并且不需要高压H 2气体和高温,因此显着降低了成本。该方法可以替代当前的 Mg(BH 4 ) 2合成工艺。








































 京公网安备 11010802027423号
京公网安备 11010802027423号