Tetrahedron Letters ( IF 1.5 ) Pub Date : 2021-12-30 , DOI: 10.1016/j.tetlet.2021.153625 Taiki Mochizuki 1 , Nanami Hoshino 1 , Aki Sato 1 , Teruo Beppu 1 , Hiroshi Katagiri 1, 2
|
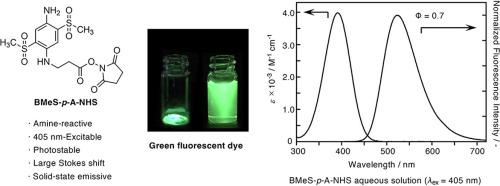
Here, we describe the synthesis and properties of BMeS-p-A-NHS as a sulfonylaniline-based fluorescent labeling reagent. Introduction of a carboxyl group into the amino group of the sulfonylaniline skeleton was achieved by the ring-opening reaction of β-propiolactone. This small labeling reagent was water-soluble, photostable, solid-state emissive, and suitable for excitation with a versatile 405-nm laser diode, emitting green fluorescence with a large Stokes shift at 527 nm. The succinimidyl ester reactive group facilitated conjugation with substrates containing amino groups.
中文翻译:

BMeS-pA 琥珀酰亚胺酯作为磺酰苯胺染料标记试剂
在这里,我们描述了 BMeS- p -A-NHS 作为磺酰苯胺基荧光标记试剂的合成和性质。通过β-丙内酯的开环反应将羧基引入磺酰苯胺骨架的氨基中。这种小型标记试剂是水溶性的、光稳定的、固态发射的,适合用多功能 405 nm 激光二极管激发,在 527 nm 处发出具有大斯托克斯位移的绿色荧光。琥珀酰亚胺酯反应性基团促进与含有氨基的底物结合。






























 京公网安备 11010802027423号
京公网安备 11010802027423号