当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulating Angiogenesis by Proteomimetics of Vascular Endothelial Growth Factor
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-30 , DOI: 10.1021/jacs.1c09571
Sami Abdulkadir 1 , Chunpu Li 1, 2 , Wei Jiang 1, 3 , Xue Zhao 1 , Peng Sang 1 , Lulu Wei 1 , Yong Hu 3 , Qi Li 2 , Jianfeng Cai 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-30 , DOI: 10.1021/jacs.1c09571
Sami Abdulkadir 1 , Chunpu Li 1, 2 , Wei Jiang 1, 3 , Xue Zhao 1 , Peng Sang 1 , Lulu Wei 1 , Yong Hu 3 , Qi Li 2 , Jianfeng Cai 1
Affiliation
|
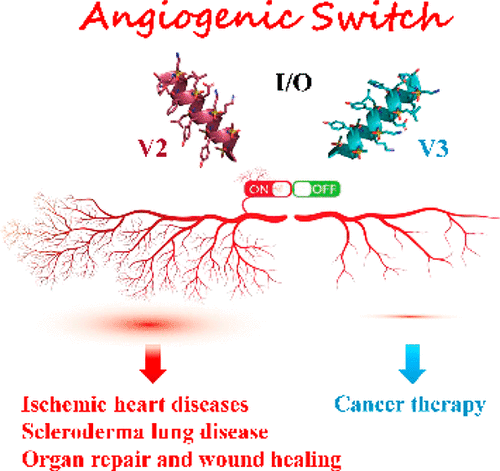
Angiogenesis, formation of new blood vessels from the existing vascular network, is a hallmark of cancer cells that leads to tumor vascular proliferation and metastasis. This process is mediated through the binding interaction of VEGF-A with VEGF receptors. However, the balance between pro-angiogenic and anti-angiogenic effect after ligand binding yet remains elusive and is therefore challenging to manipulate. To interrogate this interaction, herein we designed a few sulfono-γ-AA peptide based helical peptidomimetics that could effectively mimic a key binding interface on VEGF (helix-α1) for VEGFR recognition. Intriguingly, although both sulfono-γ-AA peptide sequences V2 and V3 bound to VEGF receptors tightly, in vitro angiogenesis assays demonstrated that V3 potently inhibited angiogenesis, whereas V2 activated angiogenesis effectively instead. Our findings suggested that this distinct modulation of angiogenesis might be due to the result of selective binding of V2 to VEGFR-1 and V3 to VEGFR-2, respectively. These molecules thus provide us a key to switch the angiogenic signaling, a biological process that balances the effects of pro-angiogenic and anti-angiogenic factors, where imbalances lead to several diseases including cancer. In addition, both V2 and V3 exhibited remarkable stability toward proteolytic hydrolysis, suggesting that V2 and V3 are promising therapeutic agents for the intervention of disease conditions arising due to angiogenic imbalances and could also be used as novel molecular switching probes to interrogate the mechanism of VEGFR signaling. The findings also further demonstrated the potential of sulfono-γ-AA peptides to mimic the α-helical domain for protein recognition and modulation of protein–protein interactions.
中文翻译:

通过血管内皮生长因子的蛋白质模拟调节血管生成
血管生成,即从现有血管网络形成新血管,是导致肿瘤血管增殖和转移的癌细胞的标志。该过程是通过 VEGF-A 与 VEGF 受体的结合相互作用介导的。然而,配体结合后促血管生成和抗血管生成作用之间的平衡仍然难以捉摸,因此难以操纵。为了探究这种相互作用,本文中我们设计了一些基于磺基-γ-AA肽的螺旋拟肽,其可以有效地模拟VEGF(螺旋-α1)上用于VEGFR识别的关键结合界面。有趣的是,虽然磺基-γ-AA肽序列V2和V3都与VEGF受体紧密结合,但体外血管生成测定表明V3有效抑制血管生成,而V2反而有效激活血管生成。我们的研究结果表明,血管生成的这种独特调节可能是由于V2与 VEGFR-1 和V3分别与 VEGFR-2 选择性结合的结果。因此,这些分子为我们提供了切换血管生成信号传导的关键,血管生成信号传导是一种平衡促血管生成因子和抗血管生成因子作用的生物过程,其中不平衡会导致包括癌症在内的多种疾病。此外, V2和V3都对蛋白水解表现出显着的稳定性,这表明V2和V3是干预因血管生成失衡而引起的疾病的有前途的治疗剂,并且也可以用作新型分子转换探针来探究VEGFR的机制发信号。 研究结果还进一步证明了磺基-γ-AA 肽模拟 α-螺旋结构域用于蛋白质识别和蛋白质-蛋白质相互作用调节的潜力。
更新日期:2022-01-12
中文翻译:

通过血管内皮生长因子的蛋白质模拟调节血管生成
血管生成,即从现有血管网络形成新血管,是导致肿瘤血管增殖和转移的癌细胞的标志。该过程是通过 VEGF-A 与 VEGF 受体的结合相互作用介导的。然而,配体结合后促血管生成和抗血管生成作用之间的平衡仍然难以捉摸,因此难以操纵。为了探究这种相互作用,本文中我们设计了一些基于磺基-γ-AA肽的螺旋拟肽,其可以有效地模拟VEGF(螺旋-α1)上用于VEGFR识别的关键结合界面。有趣的是,虽然磺基-γ-AA肽序列V2和V3都与VEGF受体紧密结合,但体外血管生成测定表明V3有效抑制血管生成,而V2反而有效激活血管生成。我们的研究结果表明,血管生成的这种独特调节可能是由于V2与 VEGFR-1 和V3分别与 VEGFR-2 选择性结合的结果。因此,这些分子为我们提供了切换血管生成信号传导的关键,血管生成信号传导是一种平衡促血管生成因子和抗血管生成因子作用的生物过程,其中不平衡会导致包括癌症在内的多种疾病。此外, V2和V3都对蛋白水解表现出显着的稳定性,这表明V2和V3是干预因血管生成失衡而引起的疾病的有前途的治疗剂,并且也可以用作新型分子转换探针来探究VEGFR的机制发信号。 研究结果还进一步证明了磺基-γ-AA 肽模拟 α-螺旋结构域用于蛋白质识别和蛋白质-蛋白质相互作用调节的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号