当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Hydroxylation of α,α-Disubstituted N-tert-Butanesulfinyl Ketimines with Molecular Oxygen: Stereoselective Synthesis of α-Tertiary Hydroxyimines
Organic Letters ( IF 4.9 ) Pub Date : 2021-12-30 , DOI: 10.1021/acs.orglett.1c04198 Nuermaimaiti Yisimayili 1, 2 , Hui Liu 1 , Yun Yao 3 , Chong-Dao Lu 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2021-12-30 , DOI: 10.1021/acs.orglett.1c04198 Nuermaimaiti Yisimayili 1, 2 , Hui Liu 1 , Yun Yao 3 , Chong-Dao Lu 1, 3
Affiliation
|
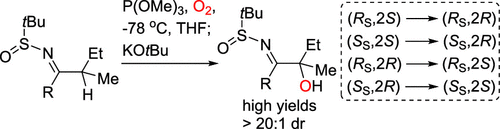
α-Tertiary hydroxyimines were stereoselectively synthesized from enantioenriched N-tert-butanesulfinyl ketimines using potassium tert-butoxide, molecular oxygen, and trimethyl phosphite. The stereoselective hydroxylation of acyclic ketimines bearing two sterically similar α-substituents was achieved by controlling the geometry of the metalloenamine intermediates and the facial selectivity of hydroxylation. The synthetic utility of the resulting α-tertiary hydroxyimines was demonstrated through the successful diastereoselective synthesis of highly substituted β-amino alcohols.
中文翻译:

α,α-二取代的 N-叔丁亚磺酰基酮亚胺与分子氧的 α-羟基化:α-叔羟基亚胺的立体选择性合成
使用叔丁醇钾、分子氧和亚磷酸三甲酯,由对映体富集的N-叔丁烷亚磺酰基酮亚胺立体选择性合成 α-叔羟基亚胺。通过控制金属烯胺中间体的几何形状和羟基化的面选择性,实现了带有两个空间相似的α-取代基的无环酮亚胺的立体选择性羟基化。通过成功地非对映选择性合成高度取代的 β-氨基醇,证明了所得 α-叔羟基亚胺的合成效用。
更新日期:2022-01-21
中文翻译:

α,α-二取代的 N-叔丁亚磺酰基酮亚胺与分子氧的 α-羟基化:α-叔羟基亚胺的立体选择性合成
使用叔丁醇钾、分子氧和亚磷酸三甲酯,由对映体富集的N-叔丁烷亚磺酰基酮亚胺立体选择性合成 α-叔羟基亚胺。通过控制金属烯胺中间体的几何形状和羟基化的面选择性,实现了带有两个空间相似的α-取代基的无环酮亚胺的立体选择性羟基化。通过成功地非对映选择性合成高度取代的 β-氨基醇,证明了所得 α-叔羟基亚胺的合成效用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号