当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spiropyran Photoisomerization Dynamics in Multiresponsive Hydrogels
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-29 , DOI: 10.1021/jacs.1c08778
Amos Meeks 1 , Michael M Lerch 1, 2 , Thomas B H Schroeder 1 , Ankita Shastri 3 , Joanna Aizenberg 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-29 , DOI: 10.1021/jacs.1c08778
Amos Meeks 1 , Michael M Lerch 1, 2 , Thomas B H Schroeder 1 , Ankita Shastri 3 , Joanna Aizenberg 1, 3
Affiliation
|
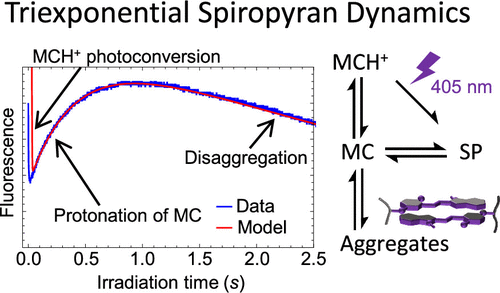
Light-responsive, spiropyran-functionalized hydrogels have been used to create reversibly photoactuated structures for applications ranging from microfluidics to nonlinear optics. Tailoring a spiropyran-functionalized hydrogel system for a particular application requires an understanding of how co-monomer composition affects the switching dynamics of the spiropyran chromophore. Such gels are frequently designed to be responsive to different stimuli such as light, temperature, and pH. The coupling of these influences can significantly alter spiropyran behavior in ways not currently well understood. To better understand the influence of responsive co-monomers on the spiropyran isomerization dynamics, we use UV–vis spectroscopy and time-dependent fluorescence intensity measurements to study spiropyran-modified hydrogels polymerized from four common hydrogel precursors of different pH and temperature responsivity: acrylamide, acrylic acid, N-isopropylacrylamide, and 2-(dimethylamino)ethyl methacrylate. In acidic and neutral gels, we observe unusual nonmonotonic, triexponential fluorescence dynamics under 405 nm irradiation that cannot be explicated by either the established spiropyran–merocyanine interconversion model or hydrolysis. To explain these results, we introduce an analytical model of spiropyran interconversions that includes H-aggregated merocyanine and its light-triggered disaggregation under 405 nm irradiation. This model provides an excellent fit to the observed fluorescence dynamics and elucidates exactly how creating an acidic internal gel environment promotes the fast and complete conversion of the hydrophilic merocyanine speciesto the hydrophobic spiropyran form, which is desired in most light-sensitive hydrogel actuators. This can be achieved by incorporating acrylic acid monomers and by minimizing the aggregate concentration. Beyond spiropyran-functionalized gel actuators, these conclusions are particularly critical for nonlinear optical computing applications.
中文翻译:

多响应水凝胶中的螺吡喃光异构化动力学
光响应性螺吡喃功能化水凝胶已被用于创建可逆光驱动结构,用于从微流体到非线性光学的应用。为特定应用定制螺吡喃功能化水凝胶系统需要了解共聚单体组成如何影响螺吡喃发色团的转换动力学。这种凝胶通常被设计成对不同的刺激(如光、温度和 pH 值)有反应。这些影响的耦合可以显着改变螺吡喃的行为方式,目前尚不为人所知。为了更好地了解响应共聚单体对螺吡喃异构化动力学的影响,N-异丙基丙烯酰胺和甲基丙烯酸2-(二甲氨基)乙酯。在酸性和中性凝胶中,我们观察到在 405 nm 照射下不寻常的非单调三指数荧光动力学,这不能通过已建立的螺吡喃-部花青相互转化模型或水解来解释。为了解释这些结果,我们介绍了螺吡喃相互转化的分析模型,其中包括 H-聚集部花青及其在 405 nm 照射下的光触发解聚。该模型非常适合观察到的荧光动力学,并准确阐明了创建酸性内部凝胶环境如何促进亲水部花青物质快速和完全转化为疏水螺吡喃形式,这是大多数光敏水凝胶致动器所需的。这可以通过加入丙烯酸单体和最小化聚集体浓度来实现。除了螺吡喃功能化凝胶致动器之外,这些结论对于非线性光学计算应用尤其重要。
更新日期:2022-01-12
中文翻译:

多响应水凝胶中的螺吡喃光异构化动力学
光响应性螺吡喃功能化水凝胶已被用于创建可逆光驱动结构,用于从微流体到非线性光学的应用。为特定应用定制螺吡喃功能化水凝胶系统需要了解共聚单体组成如何影响螺吡喃发色团的转换动力学。这种凝胶通常被设计成对不同的刺激(如光、温度和 pH 值)有反应。这些影响的耦合可以显着改变螺吡喃的行为方式,目前尚不为人所知。为了更好地了解响应共聚单体对螺吡喃异构化动力学的影响,N-异丙基丙烯酰胺和甲基丙烯酸2-(二甲氨基)乙酯。在酸性和中性凝胶中,我们观察到在 405 nm 照射下不寻常的非单调三指数荧光动力学,这不能通过已建立的螺吡喃-部花青相互转化模型或水解来解释。为了解释这些结果,我们介绍了螺吡喃相互转化的分析模型,其中包括 H-聚集部花青及其在 405 nm 照射下的光触发解聚。该模型非常适合观察到的荧光动力学,并准确阐明了创建酸性内部凝胶环境如何促进亲水部花青物质快速和完全转化为疏水螺吡喃形式,这是大多数光敏水凝胶致动器所需的。这可以通过加入丙烯酸单体和最小化聚集体浓度来实现。除了螺吡喃功能化凝胶致动器之外,这些结论对于非线性光学计算应用尤其重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号