当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A novel domino approach for synthesis of indolyl tetrahydropyrano[4,3-c]pyrazole derivatives as anticancer agents
Tetrahedron ( IF 2.1 ) Pub Date : 2016-08-31 00:59:43 Fu-Qiang Wang, Hui Yang, Bin He, Yong-Kang Jia, Shi-Yao Meng, Chao Zhang, Hong-Min Liu, Feng-Wu Liu
Tetrahedron ( IF 2.1 ) Pub Date : 2016-08-31 00:59:43 Fu-Qiang Wang, Hui Yang, Bin He, Yong-Kang Jia, Shi-Yao Meng, Chao Zhang, Hong-Min Liu, Feng-Wu Liu
|
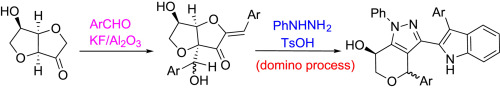
A series of indolyl substituted 1,4,6,7-tetrahydropyrano[4,3-c]pyrazole derivatives were synthesized via a domino method based on the 1,4:3,6-Dianhydrofructose. The mechanism was proposed and proved by calculation with a density functional theory (DFT), which includes formation of phenylhydrazone, furanone ring opening, Fischer indole synthesis, pyrazole ring closing and a furan ring expansion. In vitro antitumor activity of all synthesized compounds against four human cancer cell lines (MCF-7, EC-109, HGC-27, and PC-3 cell lines) were then evaluated, using MTT assay. We found that most of the compounds were more potent than the positive control 5-fluorouracil. Particularly, compound 4i against HGC-27 and PC-3, 4j against EC-109 cell lines showed three fold greater activity than 5-fluorouracil, respectively. The activity of compounds 4c and 4d against MCF-7 cell line was close to that of doxorubicin.
中文翻译:

一种新颖的多米诺骨牌合成吲哚基四氢吡喃并[4,3-c]吡唑衍生物作为抗癌药的方法
基于1,4:3,6-二氢果糖,通过多米诺法合成了一系列吲哚基取代的1,4,6,7-四氢吡喃并[4,3-c]吡唑衍生物。提出并通过密度泛函理论(DFT)的计算证明了该机理,该理论包括苯hydr的形成,呋喃酮的开环,费歇尔吲哚合成,吡唑环的闭合和呋喃环的扩展。然后使用MTT分析评估所有合成化合物对四种人类癌细胞系(MCF-7,EC-109,HGC-27和PC-3细胞系)的体外抗肿瘤活性。我们发现大多数化合物比阳性对照5-氟尿嘧啶更有效。特别地,针对HGC-27和PC-3的化合物4i,针对EC-109细胞系的4j分别显示出比5-氟尿嘧啶高三倍的活性。
更新日期:2016-08-31
中文翻译:

一种新颖的多米诺骨牌合成吲哚基四氢吡喃并[4,3-c]吡唑衍生物作为抗癌药的方法
基于1,4:3,6-二氢果糖,通过多米诺法合成了一系列吲哚基取代的1,4,6,7-四氢吡喃并[4,3-c]吡唑衍生物。提出并通过密度泛函理论(DFT)的计算证明了该机理,该理论包括苯hydr的形成,呋喃酮的开环,费歇尔吲哚合成,吡唑环的闭合和呋喃环的扩展。然后使用MTT分析评估所有合成化合物对四种人类癌细胞系(MCF-7,EC-109,HGC-27和PC-3细胞系)的体外抗肿瘤活性。我们发现大多数化合物比阳性对照5-氟尿嘧啶更有效。特别地,针对HGC-27和PC-3的化合物4i,针对EC-109细胞系的4j分别显示出比5-氟尿嘧啶高三倍的活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号