当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal Structure of Na2V2(PO4)3, an Intriguing Phase Spotted in the Na3V2(PO4)3–Na1V2(PO4)3 System
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-12-29 , DOI: 10.1021/acs.chemmater.1c04033
Sunkyu Park 1, 2, 3 , Ziliang Wang 4 , Zeyu Deng 4 , Iona Moog 3 , Pieremanuele Canepa 4, 5 , François Fauth 6 , Dany Carlier 2, 7 , Laurence Croguennec 2, 7 , Christian Masquelier 1, 7 , Jean-Noël Chotard 1, 7
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-12-29 , DOI: 10.1021/acs.chemmater.1c04033
Sunkyu Park 1, 2, 3 , Ziliang Wang 4 , Zeyu Deng 4 , Iona Moog 3 , Pieremanuele Canepa 4, 5 , François Fauth 6 , Dany Carlier 2, 7 , Laurence Croguennec 2, 7 , Christian Masquelier 1, 7 , Jean-Noël Chotard 1, 7
Affiliation
|
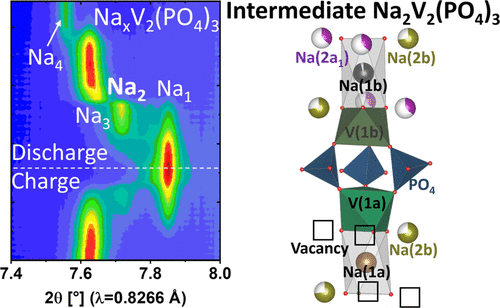
The Na superionic conductor (NASICON) Na3V2(PO4)3 is an important positive electrode material for Na-ion batteries. Here, we investigate the mechanisms of phase transition in NaxV2(PO4)3 (1 ≤ x ≤ 4) upon nonequilibrium battery cycling. Unlike the widely believed two-phase reaction in a Na3V2(PO4)3–Na1V2(PO4)3 system, we determine, for the first time, the structure of a recently reported intermediate Na2V2(PO4)3 phase using operando synchrotron X-ray diffraction. Density functional theory calculations further support the existence of the Na2V2(PO4)3 phase. We propose two possible crystal structures of Na2V2(PO4)3 analyzed by Rietveld refinement. The two structure models with the space groups P21/c or P2/c for the new intermediate Na2V2(PO4)3 phase show similar unit cell parameters but different atomic arrangements, including vanadium charge ordering. As the appearance of the intermediate Na2V2(PO4)3 phase is accompanied by symmetry reduction, Na(1) and Na(2) sites split into several positions in Na2V2(PO4)3, in which one of the splitting Na(2) position is found to be a vacancy, whereas the Na(1) positions are almost fully filled. The intermediate Na2V2(PO4)3 phase reduces the lattice mismatch between Na3V2(PO4)3 and Na1V2(PO4)3 phases, facilitating a fast phase transition. This work paves the way for a better understanding of great rate capabilities of Na3V2(PO4)3.
中文翻译:

Na2V2(PO4)3 的晶体结构,这是在 Na3V2(PO4)3–Na1V2(PO4)3 系统中发现的一个有趣的相
Na超离子导体(NASICON) Na 3 V 2 (PO 4 ) 3是钠离子电池的重要正极材料。在这里,我们研究了非平衡电池循环时Na x V 2 (PO 4 ) 3 (1 ≤ x ≤ 4)的相变机制。与广泛认为的 Na 3 V 2 (PO 4 ) 3 –Na 1 V 2 (PO 4 ) 3系统中的两相反应不同,我们首次确定了最近报道的中间体 Na 的结构2 V 2 (PO 4 ) 3相使用操作同步加速器 X 射线衍射。密度泛函理论计算进一步支持Na 2 V 2 (PO 4 ) 3相的存在。我们提出了通过 Rietveld 精修分析的两种可能的 Na 2 V 2 (PO 4 ) 3晶体结构。与空间群两个结构模型P 2 1 / ç或P 2 / ç为新中间体的Na 2 V 2(PO 4) 3相显示相似的晶胞参数但不同的原子排列,包括钒电荷排序。由于中间 Na 2 V 2 (PO 4 ) 3相的出现伴随着对称性降低,Na(1) 和 Na(2) 位点在 Na 2 V 2 (PO 4 ) 3中分裂成多个位置,其中一个分裂的 Na(2) 位置被发现是一个空缺,而 Na(1) 位置几乎被完全填满。中间的 Na 2 V 2 (PO 4 ) 3相减少了 Na 3 V之间的晶格失配2 (PO 4 ) 3和Na 1 V 2 (PO 4 ) 3相,促进快速相变。这项工作为更好地理解 Na 3 V 2 (PO 4 ) 3的高倍率能力铺平了道路。
更新日期:2022-01-11
中文翻译:

Na2V2(PO4)3 的晶体结构,这是在 Na3V2(PO4)3–Na1V2(PO4)3 系统中发现的一个有趣的相
Na超离子导体(NASICON) Na 3 V 2 (PO 4 ) 3是钠离子电池的重要正极材料。在这里,我们研究了非平衡电池循环时Na x V 2 (PO 4 ) 3 (1 ≤ x ≤ 4)的相变机制。与广泛认为的 Na 3 V 2 (PO 4 ) 3 –Na 1 V 2 (PO 4 ) 3系统中的两相反应不同,我们首次确定了最近报道的中间体 Na 的结构2 V 2 (PO 4 ) 3相使用操作同步加速器 X 射线衍射。密度泛函理论计算进一步支持Na 2 V 2 (PO 4 ) 3相的存在。我们提出了通过 Rietveld 精修分析的两种可能的 Na 2 V 2 (PO 4 ) 3晶体结构。与空间群两个结构模型P 2 1 / ç或P 2 / ç为新中间体的Na 2 V 2(PO 4) 3相显示相似的晶胞参数但不同的原子排列,包括钒电荷排序。由于中间 Na 2 V 2 (PO 4 ) 3相的出现伴随着对称性降低,Na(1) 和 Na(2) 位点在 Na 2 V 2 (PO 4 ) 3中分裂成多个位置,其中一个分裂的 Na(2) 位置被发现是一个空缺,而 Na(1) 位置几乎被完全填满。中间的 Na 2 V 2 (PO 4 ) 3相减少了 Na 3 V之间的晶格失配2 (PO 4 ) 3和Na 1 V 2 (PO 4 ) 3相,促进快速相变。这项工作为更好地理解 Na 3 V 2 (PO 4 ) 3的高倍率能力铺平了道路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号