当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Epoxide Electroreduction
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-29 , DOI: 10.1021/jacs.1c11791 Cheng Huang 1 , Wan Ma 1 , Xuelian Zheng 1 , Minghao Xu 2 , Xiaotian Qi 2 , Qingquan Lu 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-29 , DOI: 10.1021/jacs.1c11791 Cheng Huang 1 , Wan Ma 1 , Xuelian Zheng 1 , Minghao Xu 2 , Xiaotian Qi 2 , Qingquan Lu 1, 3
Affiliation
|
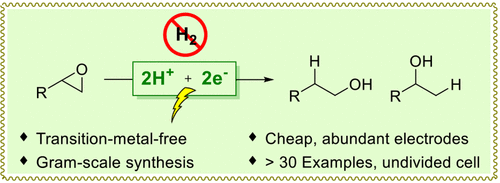
Selective hydrogenation of epoxides would be a direct and powerful approach for alcohol synthesis, but it has proven to be elusive. Here, electrochemically epoxide hydrogenation using electrons and protons as reductants is reported. A wide range of primary, secondary, and tertiary alcohols can be achieved through selective Markovnikov or anti-Markovnikov ring opening in the absence of transition metals. Mechanistic investigations revealed that the regioselectivity is controlled by the thermodynamic stabilities of the in situ generated benzyl radicals for aryl-substituted epoxides and the kinetic tendency for Markovnikov selective ring opening for alkyl-substituted epoxides.
中文翻译:

环氧化物电还原
环氧化物的选择性加氢将是一种直接且有效的醇合成方法,但已被证明是难以捉摸的。在这里,报道了使用电子和质子作为还原剂的电化学环氧化物加氢。在没有过渡金属的情况下,可以通过选择性马尔科夫尼科夫或反马尔科夫尼科夫开环来获得范围广泛的伯醇、仲醇和叔醇。机理研究表明,区域选择性受芳基取代环氧化物原位生成苄基自由基的热力学稳定性和烷基取代环氧化物马尔科夫尼科夫选择性开环的动力学趋势控制。
更新日期:2022-01-26
中文翻译:

环氧化物电还原
环氧化物的选择性加氢将是一种直接且有效的醇合成方法,但已被证明是难以捉摸的。在这里,报道了使用电子和质子作为还原剂的电化学环氧化物加氢。在没有过渡金属的情况下,可以通过选择性马尔科夫尼科夫或反马尔科夫尼科夫开环来获得范围广泛的伯醇、仲醇和叔醇。机理研究表明,区域选择性受芳基取代环氧化物原位生成苄基自由基的热力学稳定性和烷基取代环氧化物马尔科夫尼科夫选择性开环的动力学趋势控制。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号