Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-12-28 , DOI: 10.1016/j.cej.2021.134364 Yao Tang 1 , Xiaoyu Zhou 1 , Ming Lei 1 , Huimin Wang 1 , Anqi Lu 1 , Guihua Zhang 2 , Lihua Zhu 2 , Kangle Lv 1 , Heqing Tang 1
|
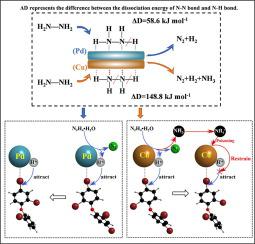
It is still urgently needed to clarify how the reductive dehalogenation is accelerated by the interaction between the catalyst and the reducing agent. In the present work, both Pd and Cu were observed to induce the debromination of 2,2’,4,4’-tetrabromodiphenyl ether (BDE47) with hydrazine hydrate (N2H4•H2O) as reducing agent. In the case of Pd system, all the added BDE47 was debrominated to bromine-free products within 20 min. However, much different debromination behaviors of BDE47 were observed when Cu was used as the catalyst: in the initial stage the debromination efficiency of BDE47 could reach up to 55.3% within 0.5 min; nevertheless the complete debromination could not be achieved by prolonging reaction time. With the aid of DFT calculations, the above-mentioned great differences were attributed to the affinity of Pd to both H and N atoms, and strong Cu-N coordination existed between Cu and the adsorbed hydrazine molecules. The dissociation energy for the cleavage of N-H and N-N bonds of N2H4•H2O adsorbed on Cu was very low, and hence the debromination of BDE47 was ultra-fast due to the spontaneously cleavage of N-H bond but it was inhibited at the later stage by the generated ammonium species (poisoning effect to the Cu catalyst) due to N-N bond cleavage. In contrast, the dissociation energy for the cleavage of N-H bond on the surface of Pd was higher and no ammonium was detected. These guarantee the continuously fast and complete debromination of BDE47 and its intermediates. These new insights to the catalytic mechanism through the interaction between the catalyst and the reducing agent will help to construct an efficient system to remove halogenated organic pollutants.
中文翻译:

以肼为还原剂高效催化脱溴四溴二苯醚:催化剂与还原剂相互作用的作用
如何通过催化剂与还原剂的相互作用加速还原脱卤,仍亟待阐明。在目前的工作中,观察到 Pd 和 Cu 均诱导 2,2',4,4'-四溴二苯醚 (BDE47) 与水合肼 (N 2 H 4 •H 2O) 作为还原剂。在 Pd 系统的情况下,所有添加的 BDE47 都在 20 分钟内脱溴为无溴产品。然而,当以Cu为催化剂时,BDE47的脱溴行为有很大不同:在初始阶段,BDE47的脱溴效率在0.5分钟内可达55.3%;然而,通过延长反应时间不能实现完全脱溴。借助 DFT 计算,上述巨大差异归因于 Pd 对 H 和 N 原子的亲和力,以及 Cu 与吸附的肼分子之间存在强的 Cu-N 配位。N 2 H 4 •H 2 NH 和NN 键断裂的解离能由于 NH 键的自发裂解,吸附在 Cu 上的 O 非常低,因此 BDE47 的脱溴速度超快,但在后期由于 NN 产生的铵物种(对 Cu 催化剂的中毒效应)抑制了脱溴键断裂。相比之下,Pd 表面 NH 键断裂的解离能更高,未检测到铵。这些保证了 BDE47 及其中间体的持续快速和完全脱溴。这些通过催化剂和还原剂之间相互作用对催化机制的新见解将有助于构建一个有效的系统来去除卤化有机污染物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号