European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-12-25 , DOI: 10.1016/j.ejmech.2021.114071 Eurico Lima 1 , Andreia G Barroso 2 , Margarida A Sousa 2 , Octávio Ferreira 3 , Renato E Boto 3 , José R Fernandes 2 , Paulo Almeida 3 , Samuel M Silvestre 4 , Adriana O Santos 3 , Lucinda V Reis 2
|
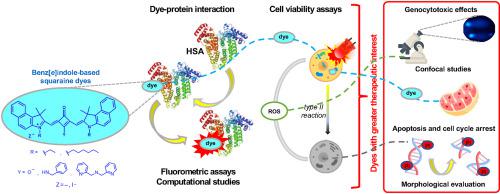
Squaraine dyes are a family of compounds known for their relevant photophysical and photochemical properties potentially useful as photosensitizing agents. Since pyridines have been introduced into the skeleton of several families of compounds to enhance their pharmacological activity, and this approach had not yet been performed on squaraines, novel dyes derived from benz[e]indole functionalized with picolyl- and dipicolylamine and N-ethyl and -hexyl chains were designed and synthesized. After being fully characterized, their interaction with human albumin was in vitro and in silico evaluated. Dyes were further assessed for their phototoxicity activity, and the most interesting ones were studied regarding cell localization and induction of morphological cell changes, genotoxicity, apoptosis and cell cycle arrest. The molecules with N-ethyl chains showed the greatest in vitro light-dependent cytotoxic effects, particularly the zwitterionic squaraine dye and the one bearing a single pyridine unit, which also exhibited a more significant interaction with human albumin. Phenotypically, the cells incubated with these squaraines became smaller and rounded after irradiation, the effects varying with the tested concentration. Genotoxic effects were observed even without irradiation, being more evident for the N-ethyl picolylamine-derived dye. The fluorescence emitted by Rhodamine 123 largely coincided with that emitted by the dyes, suggesting that they are found preferentially in mitochondria. After irradiation, an increase in the subG1 population was verified by propidium iodide-staining analysis by flow cytometry, indicative of cell death by apoptosis.
中文翻译:

吡啶甲胺官能化苯并[e]吲哚方酸染料:作为潜在抗癌光疗剂的合成方法、表征和体外功效
方酸染料是一类化合物,以其相关的光物理和光化学特性而闻名,这些特性可能可用作光敏剂。由于吡啶已被引入几个化合物家族的骨架以增强它们的药理活性,而这种方法尚未在方酸上进行,因此衍生自苯并[ e ]吲哚的新型染料用吡啶甲基和二吡啶胺以及N-乙基和-己基链的设计和合成。在完全表征后,它们与人白蛋白的相互作用是在体外和计算机中进行的评估。进一步评估了染料的光毒性活性,研究了最有趣的染料在细胞定位和诱导形态细胞变化、遗传毒性、细胞凋亡和细胞周期停滞方面的作用。具有N-乙基链的分子表现出最大的体外光依赖性细胞毒作用,特别是两性离子方酸染料和带有单个吡啶单元的染料,其也表现出与人白蛋白更显着的相互作用。在表型上,与这些方酸一起孵育的细胞在照射后变得更小和更圆,效果随测试浓度而变化。即使没有照射也观察到基因毒性效应,对于N-乙基吡啶甲胺衍生染料。罗丹明 123 发出的荧光在很大程度上与染料发出的荧光一致,表明它们优先存在于线粒体中。照射后,通过流式细胞仪进行的碘化丙啶染色分析证实了亚 G1 群的增加,表明细胞凋亡导致细胞死亡。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号