当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of a cis-4-aminopiperidine-3-carboxylic acid (cis-APiC) residue on mixed-helical folding of unnatural peptides
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-12-17 , DOI: 10.1039/d1ob02223g Sunglim Choi 1 , Jihyun Shim 1 , Philjae Kang 1 , Soo Hyuk Choi 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-12-17 , DOI: 10.1039/d1ob02223g Sunglim Choi 1 , Jihyun Shim 1 , Philjae Kang 1 , Soo Hyuk Choi 1
Affiliation
|
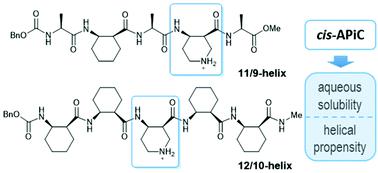
The α/β-peptide 11/9-helix and the β-peptide 12/10-helix belong to “mixed” helices, in which two types of hydrogen bonds with opposite directionality alternate along the helical axis. cis-2-Aminocyclohexanecarboxylic acid (cis-ACHC) is known to promote these mixed helices and stabilize the helical propensity more than other acyclic β-residues. Application of a mixed-helical backbone still requires sufficient solubility in aqueous solution. In this regard, we chose cis-4-aminopiperidine-3-carboxylic acid (cis-APiC) as a foldamer building block that can provide both sufficient aqueous solubility and mixed-helical propensity. Conformational analyses of α/β- and β-peptides containing a cis-APiC residue by circular dichroism spectroscopy and single-crystal X-ray crystallography suggest that the incorporation of cis-APiC instead of cis-ACHC can enhance the aqueous solubility of the mixed-helical peptides without any adverse effect on helical folding. In addition, the ratio between right- and left-handed 12/10-helices of β-peptides can be rationalized by relative energies between the local conformations of the cis-APiC residue.
中文翻译:

顺式-4-氨基哌啶-3-羧酸 (cis-APiC) 残基对非天然肽混合螺旋折叠的影响
α/β-肽11/9-螺旋和β-肽12/10-螺旋属于“混合”螺旋,其中两种方向相反的氢键沿螺旋轴交替。与其他无环 β-残基相比,顺式-2-氨基环己烷羧酸 ( cis -ACHC) 已知能促进这些混合螺旋并更稳定螺旋倾向。混合螺旋骨架的应用仍然需要在水溶液中具有足够的溶解度。在这方面,我们选择了顺式-4-氨基哌啶-3-羧酸(顺式-APiC)作为折叠体结构单元,它可以提供足够的水溶性和混合螺旋倾向。含有顺式的α/β-和β-肽的构象分析圆二色光谱和单晶X射线晶体学的-APiC残基表明,顺式-APiC而不是顺式-ACHC的掺入可以增强混合螺旋肽的水溶性,而对螺旋折叠没有任何不利影响。此外,β-肽的右手和左手 12/10-螺旋之间的比率可以通过cis -APiC 残基的局部构象之间的相对能量来合理化。
更新日期:2021-12-24
中文翻译:

顺式-4-氨基哌啶-3-羧酸 (cis-APiC) 残基对非天然肽混合螺旋折叠的影响
α/β-肽11/9-螺旋和β-肽12/10-螺旋属于“混合”螺旋,其中两种方向相反的氢键沿螺旋轴交替。与其他无环 β-残基相比,顺式-2-氨基环己烷羧酸 ( cis -ACHC) 已知能促进这些混合螺旋并更稳定螺旋倾向。混合螺旋骨架的应用仍然需要在水溶液中具有足够的溶解度。在这方面,我们选择了顺式-4-氨基哌啶-3-羧酸(顺式-APiC)作为折叠体结构单元,它可以提供足够的水溶性和混合螺旋倾向。含有顺式的α/β-和β-肽的构象分析圆二色光谱和单晶X射线晶体学的-APiC残基表明,顺式-APiC而不是顺式-ACHC的掺入可以增强混合螺旋肽的水溶性,而对螺旋折叠没有任何不利影响。此外,β-肽的右手和左手 12/10-螺旋之间的比率可以通过cis -APiC 残基的局部构象之间的相对能量来合理化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号