Dyes and Pigments ( IF 4.1 ) Pub Date : 2021-12-24 , DOI: 10.1016/j.dyepig.2021.110056 Yan-Ru Huang , Sheng-Huei Hsiao
|
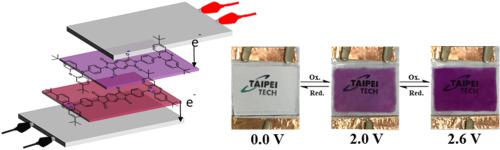
Five arylene diimides containing N-phenylphenothiazine or N-phenyl-3,7-di-tert-butylphenothiazine units as N-substituents, coded as PTZ-PMDI, PTZ-NTDI, tBuPTZ-PMDI, tBuPTZ-NTDI and tBuPTZ-PTDI, were synthesized from condensation of N-(4-aminophenyl)phenothiazine and N-(4-aminophenyl)-3,7-di-tert-butylphenothiazine with pyromellitic dianhydride (PMDA), naphthalene-1,4,5,8-tetracarboxylic dianhydride (NTDA) and perylene-3,4,9,10-tetracarboxylic dianhydride (PTDA), respectively. Incorporation of tert-butyl substituents on the PTZ active sites reduces the oxidation potential and increases the redox stability and solubility of the PTZ-diimides. PTZ-PMDI and PTZ-NTDI without tert-butyl substituents on the PTZ units are insoluble in suitable organic solvents, therefore the characterization of their electrochemical and electrochromic properties is unavailable. All the tBuPTZ-diimide compounds were electrochemically active and underwent reversible oxidation and quasi-reversible reduction as evidenced by cyclic voltammetry studies. These diimide compounds changed colors when they were reduced and oxidized, demonstrating their multi-electrochromic properties. The tBuPTZ-diimides gave a colored state of pink and red upon electro-oxidation, together with a low onset bias of 0.6 V and good switching stabilities. The electrochromic devices using these diimides as electroactive compounds were constructed and their electrochromic performance was studied.
中文翻译:

N-苯基吩噻嗪单元亚芳基二亚胺染料的电化学和电致变色性能
五种含有N-苯基吩噻嗪或N-苯基-3,7-二叔丁基吩噻嗪单元作为N-取代基的亚芳基二酰亚胺,编码为PTZ-PMDI、PTZ-NTDI、tBuPTZ-PMDI、tBuPTZ-NTDI和tBuPTZ-PTDI,分别为从缩合合成ñ(4-氨基苯基)吩噻嗪和- ñ - (4-氨基苯基)-3,7-二-叔-butylphenothiazine与1,2,4,5-苯四酸二酐(PMDA),萘-1,4,5,8-四羧酸二酐(NTDA ) 和苝-3,4,9,10-四羧酸二酐 ( PTDA)), 分别。在 PTZ 活性位点上加入叔丁基取代基可降低氧化电位并增加 PTZ-二亚胺的氧化还原稳定性和溶解度。PTZ-PMDI和PTZ-NTDI不带叔PTZ 单元上的-丁基取代基不溶于合适的有机溶剂,因此无法对其电化学和电致变色特性进行表征。所有 tBuPTZ-二亚胺化合物都具有电化学活性,并经历了可逆氧化和准可逆还原,如循环伏安法研究所证明的。这些二亚胺化合物在被还原和氧化时会改变颜色,证明了它们的多电致变色特性。tBuPTZ-二亚胺在电氧化时呈现粉红色和红色的有色状态,同时具有 0.6 V 的低起始偏压和良好的开关稳定性。构建了使用这些二亚胺作为电活性化合物的电致变色器件并研究了它们的电致变色性能。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号