Food Chemistry ( IF 8.5 ) Pub Date : 2021-12-24 , DOI: 10.1016/j.foodchem.2021.131945 Rui Wang 1 , Qin-Hui Wen 2 , Xin-An Zeng 3 , Jia-Wei Lin 1 , Jian Li 1 , Fei-Yue Xu 1
|
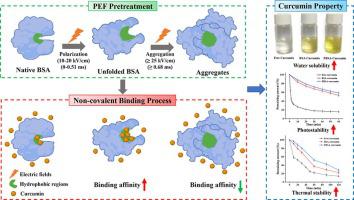
The present study investigated the effect of pulsed electric field (PEF) pretreatment on the interaction between bovine serum albumin (BSA) and curcumin. Fluorescence quenching results showed that proper PEF pretreatment significantly increased the binding affinity of curcumin and BSA, the binding constant increased by 6.77 times under the conditions of 15 kV/cm for 0.51 ms. However, at higher PEF strength (≥25 kV/cm) and longer processing time (≥0.68 ms), the binding affinity was weakened. PEF pretreatment made the protein structure more disordered and induced partial unfolding of BSA, exposing more hydrophobic regions, thereby increasing the binding affinity to curcumin. PEF-treated BSA (PBSA) possessed better encapsulation efficiency (95.19%) and loading capacity (5.25 mg/g) for curcumin, and the storage stability of curcumin were enhanced by the formation of a complex with PBSA. This study provides new insights into the design of BSA-based delivery systems for curcumin and other hydrophobic nutrients.
中文翻译:

脉冲电场预处理增强姜黄素与牛血清白蛋白的结合亲和力
本研究调查了脉冲电场 (PEF) 预处理对牛血清白蛋白 (BSA) 和姜黄素之间相互作用的影响。荧光猝灭结果表明,适当的PEF预处理显着提高了姜黄素和BSA的结合亲和力,在15 kV/cm、0.51 ms条件下结合常数提高了6.77倍。然而,在较高的 PEF 强度 (≥25 kV/cm) 和较长的处理时间 (≥0.68 ms) 下,结合亲和力减弱。PEF预处理使蛋白质结构更加无序并诱导BSA部分展开,暴露更多疏水区域,从而增加与姜黄素的结合亲和力。PEF处理的BSA(PBSA)对姜黄素具有更好的包封率(95.19%)和负载能力(5.25 mg/g),姜黄素与 PBSA 形成复合物增强了姜黄素的储存稳定性。这项研究为设计基于 BSA 的姜黄素和其他疏水性营养素递送系统提供了新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号