Cell Reports Medicine ( IF 11.7 ) Pub Date : 2021-12-21 , DOI: 10.1016/j.xcrm.2021.100457
Tamara Muliaditan 1 , Leena Halim 2 , Lynsey M Whilding 2 , Benjamin Draper 2 , Daniela Y Achkova 2 , Fahima Kausar 1 , Maya Glover 1 , Natasha Bechman 2 , Appitha Arulappu 1 , Jenifer Sanchez 3 , Katie R Flaherty 3 , Jana Obajdin 1 , Kristiana Grigoriadis 4 , Pierre Antoine 2 , Daniel Larcombe-Young 2 , Caroline M Hull 1, 2 , Richard Buus 5, 6 , Peter Gordon 2 , Anita Grigoriadis 4 , David M Davies 1, 2 , Anna Schurich 3 , John Maher 1, 2, 7, 8
|
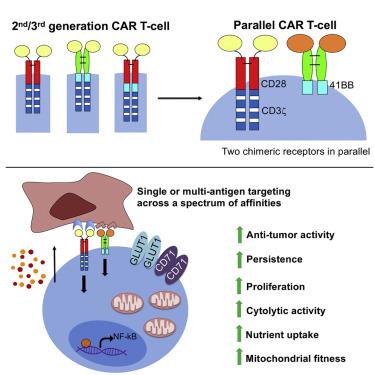
Second generation (2G) chimeric antigen receptors (CARs) contain a CD28 or 41BB co-stimulatory endodomain and elicit remarkable efficacy in hematological malignancies. Third generation (3G) CARs extend this linear blueprint by fusing both co-stimulatory units in series. However, clinical impact has been muted despite compelling evidence that co-signaling by CD28 and 41BB can powerfully amplify natural immune responses. We postulate that effective dual co-stimulation requires juxta-membrane positioning of endodomain components within separate synthetic receptors. Consequently, we designed parallel (p)CARs in which a 2G (CD28+CD3ζ) CAR is co-expressed with a 41BB-containing chimeric co-stimulatory receptor. We demonstrate that the pCAR platform optimally harnesses synergistic and tumor-dependent co-stimulation to resist T cell exhaustion and senescence, sustaining proliferation, cytokine release, cytokine signaling, and metabolic fitness upon repeated stimulation. When engineered using targeting moieties of diverse composition, affinity, and specificity, pCAR T cells consistently elicit superior anti-tumor activity compared with T cells that express traditional linear CARs.
中文翻译:

41BB 和 CD28 的协同 T 细胞信号传导可通过平行嵌合抗原受体内的膜近端定位来最佳实现
第二代 (2G) 嵌合抗原受体 (CAR) 含有 CD28 或 41BB 共刺激内域,对血液恶性肿瘤具有显着疗效。第三代 (3G) CAR 通过串联融合两个共刺激单元来扩展此线性蓝图。然而,尽管有令人信服的证据表明 CD28 和 41BB 的共同信号传导可以强有力地放大自然免疫反应,但临床影响却很有限。我们假设有效的双重共刺激需要将内域成分置于单独的合成受体内的并膜位置。因此,我们设计了平行的 (p)CAR,其中 2G (CD28+CD3z) CAR 与包含 41BB 的嵌合共刺激受体共表达。我们证明,pCAR 平台最佳地利用协同和肿瘤依赖性共刺激来抵抗 T 细胞耗竭和衰老,在重复刺激时维持增殖、细胞因子释放、细胞因子信号传导和代谢适应性。当使用不同组成、亲和力和特异性的靶向部分进行工程改造时,与表达传统线性 CAR 的 T 细胞相比,pCAR T 细胞始终能产生优异的抗肿瘤活性。


































 京公网安备 11010802027423号
京公网安备 11010802027423号