当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Spectroscopy of Oxyluciferin, the Light Emitter of the Firefly Bioluminescence
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-08-19 , DOI: 10.1021/ja904309q Panče Naumov 1 , Yutaka Ozawa 1 , Kei Ohkubo 1 , Shunichi Fukuzumi 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-08-19 , DOI: 10.1021/ja904309q Panče Naumov 1 , Yutaka Ozawa 1 , Kei Ohkubo 1 , Shunichi Fukuzumi 1
Affiliation
|
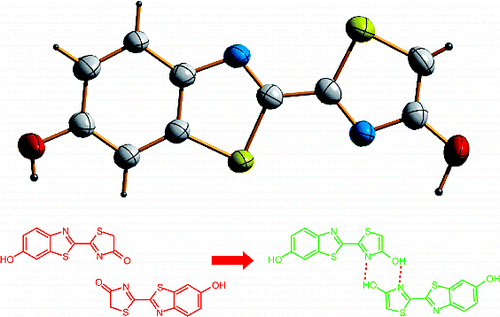
The crystal structures of the pure, unsubstituted firefly emitter oxyluciferin (OxyLH(2)) and its 5-methyl analogue (MOxyLH(2)) were determined for the first time to reveal that both molecules exist as pure trans-enol forms, enol-OxyLH(2) and enol-MOxyLH(2), assembled as head-to-tail hydrogen-bonded dimers. Their steady-state absorption and emission spectra (in solution and in the solid state) and nanosecond time-resolved fluorescence decays (in solution) were recorded and assigned to the six possible trans chemical forms of the emitter and its anions. The spectra of the pure emitter were compared to its bioluminescence and fluorescence spectra when it is complexed with luciferase from the Japanese firefly (Luciola cruciata) and interpreted in terms of the intermolecular interactions based on the structure of the emitter in the luciferase active site. The wavelengths of the emission spectral maxima of the six chemical forms of OxyLH(2) are generally in good agreement with the theoretically predicted energies of the S(0)-S(1) transitions and range from the blue to the red regions, while the respective absorption maxima range from the ultraviolet to the green regions. It was confirmed that both neutral forms, phenol-enol and phenol-keto, are blue emitters, whereas the phenolate-enol form is yellow-green emitter. The phenol-enolate form, which probably only exists as a mixture with other species, and the phenolate-enolate dianion are yellow or orange emitters with close position of their emission bands. The phenolate-keto form always emits in the red region. The concentration ratio of the different chemical species in solutions of OxyLH(2) is determined by several factors which affect the intricate triple chemical equilibrium, most notably the pH, solvent polarity, hydrogen bonding, presence of additional ions, and pi-pi stacking. Due to the stabilization of the enol group of the 4-hydroxythiazole ring by hydrogen bonding to the proximate adenosine monophosphate, which according to the density functional calculations is similar to that due to the dimerization of two enol molecules observed in the crystal, the phenolate ion of the enol tautomer, which is the predominant ground-state species within the narrow pH interval 7.44-8.14 in buffered aqueous solutions, is the most probable emitter of the yellow-green bioluminescence common for most wild-type luciferases. This conclusion is supported by the bioluminescence/fluorescence spectra and the NMR data, as well the crystal structures of OxyLH(2) and MOxyLH(2), where the conjugated acid (phenol) of the emitter exists as pure enol tautomer.
中文翻译:

氧化荧光素的结构和光谱,萤火虫生物发光的发光体
首次确定了纯的、未取代的萤火虫发射体氧化荧光素 (OxyLH(2)) 及其 5-甲基类似物 (MOxyLH(2)) 的晶体结构,以揭示这两种分子均以纯反式烯醇形式存在,烯醇- OxyLH(2) 和烯醇-MOxyLH(2),组装为头对尾的氢键二聚体。它们的稳态吸收和发射光谱(在溶液中和固态中)和纳秒时间分辨荧光衰减(在溶液中)被记录并分配给六种可能的发射体及其阴离子的反式化学形式。当纯发射体与来自日本萤火虫 (Luciola cruciata) 的荧光素酶复合时,将纯发射体的光谱与其生物发光和荧光光谱进行比较,并根据荧光素酶活性位点中发射体的结构根据分子间相互作用进行解释。OxyLH(2) 的六种化学形式的发射光谱最大值的波长通常与 S(0)-S(1) 跃迁的理论预测能量非常一致,范围从蓝色到红色区域,而各自的吸收最大值范围从紫外线到绿色区域。经证实,酚-烯醇和酚-酮这两种中性形式都是蓝色发射体,而酚盐-烯醇形式是黄绿色发射体。苯酚-烯醇化物形式,可能只与其他物种混合存在,酚盐-烯醇二价阴离子是黄色或橙色的发射体,它们的发射带位置很近。酚盐-酮形式总是在红色区域发射。OxyLH(2) 溶液中不同化学物质的浓度比由影响复杂三重化学平衡的几个因素决定,最显着的是 pH 值、溶剂极性、氢键、附加离子的存在和 pi-pi 堆叠。由于 4-羟基噻唑环的烯醇基团通过氢键与邻近的单磷酸腺苷键合而稳定,根据密度泛函计算,这类似于由于在晶体中观察到的两个烯醇分子的二聚化,酚离子烯醇互变异构体,它是窄 pH 值区间 7.44-8 内的主要基态物质。14 在缓冲水溶液中,是大多数野生型荧光素酶常见的黄绿色生物发光的最可能发射体。这一结论得到了生物发光/荧光光谱和 NMR 数据的支持,以及 OxyLH(2) 和 MOxyLH(2) 的晶体结构,其中发射体的共轭酸(苯酚)作为纯烯醇互变异构体存在。
更新日期:2009-08-19
中文翻译:

氧化荧光素的结构和光谱,萤火虫生物发光的发光体
首次确定了纯的、未取代的萤火虫发射体氧化荧光素 (OxyLH(2)) 及其 5-甲基类似物 (MOxyLH(2)) 的晶体结构,以揭示这两种分子均以纯反式烯醇形式存在,烯醇- OxyLH(2) 和烯醇-MOxyLH(2),组装为头对尾的氢键二聚体。它们的稳态吸收和发射光谱(在溶液中和固态中)和纳秒时间分辨荧光衰减(在溶液中)被记录并分配给六种可能的发射体及其阴离子的反式化学形式。当纯发射体与来自日本萤火虫 (Luciola cruciata) 的荧光素酶复合时,将纯发射体的光谱与其生物发光和荧光光谱进行比较,并根据荧光素酶活性位点中发射体的结构根据分子间相互作用进行解释。OxyLH(2) 的六种化学形式的发射光谱最大值的波长通常与 S(0)-S(1) 跃迁的理论预测能量非常一致,范围从蓝色到红色区域,而各自的吸收最大值范围从紫外线到绿色区域。经证实,酚-烯醇和酚-酮这两种中性形式都是蓝色发射体,而酚盐-烯醇形式是黄绿色发射体。苯酚-烯醇化物形式,可能只与其他物种混合存在,酚盐-烯醇二价阴离子是黄色或橙色的发射体,它们的发射带位置很近。酚盐-酮形式总是在红色区域发射。OxyLH(2) 溶液中不同化学物质的浓度比由影响复杂三重化学平衡的几个因素决定,最显着的是 pH 值、溶剂极性、氢键、附加离子的存在和 pi-pi 堆叠。由于 4-羟基噻唑环的烯醇基团通过氢键与邻近的单磷酸腺苷键合而稳定,根据密度泛函计算,这类似于由于在晶体中观察到的两个烯醇分子的二聚化,酚离子烯醇互变异构体,它是窄 pH 值区间 7.44-8 内的主要基态物质。14 在缓冲水溶液中,是大多数野生型荧光素酶常见的黄绿色生物发光的最可能发射体。这一结论得到了生物发光/荧光光谱和 NMR 数据的支持,以及 OxyLH(2) 和 MOxyLH(2) 的晶体结构,其中发射体的共轭酸(苯酚)作为纯烯醇互变异构体存在。































 京公网安备 11010802027423号
京公网安备 11010802027423号