Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Polarity Fluoroalkyl Ether Electrolyte Enables Solvation-Free Li+ Transfer for High-Rate Lithium Metal Batteries
Advanced Science ( IF 14.3 ) Pub Date : 2021-12-19 , DOI: 10.1002/advs.202104699 Liwei Dong 1, 2, 3 , Yuanpeng Liu 1 , Kechun Wen 1 , Dongjiang Chen 4 , Dewei Rao 5 , Jipeng Liu 1, 2 , Botao Yuan 1 , Yunfa Dong 1 , Ze Wu 2 , Yifang Liang 1, 2 , Mengqiu Yang 1, 2 , Jianyi Ma 6 , Chunhui Yang 2, 3 , Chuan Xia 7 , Baoyu Xia 8 , Jiecai Han 1 , Gongming Wang 9 , Zaiping Guo 10 , Weidong He 1, 4
Advanced Science ( IF 14.3 ) Pub Date : 2021-12-19 , DOI: 10.1002/advs.202104699 Liwei Dong 1, 2, 3 , Yuanpeng Liu 1 , Kechun Wen 1 , Dongjiang Chen 4 , Dewei Rao 5 , Jipeng Liu 1, 2 , Botao Yuan 1 , Yunfa Dong 1 , Ze Wu 2 , Yifang Liang 1, 2 , Mengqiu Yang 1, 2 , Jianyi Ma 6 , Chunhui Yang 2, 3 , Chuan Xia 7 , Baoyu Xia 8 , Jiecai Han 1 , Gongming Wang 9 , Zaiping Guo 10 , Weidong He 1, 4
Affiliation
|
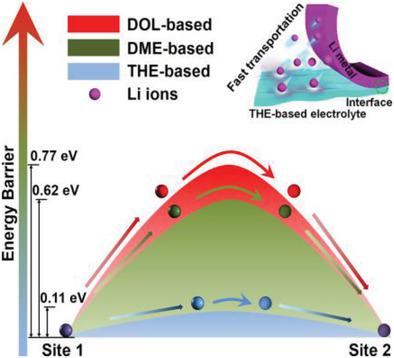
Lithium metal batteries (LMBs) have aroused extensive interest in the field of energy storage owing to the ultrahigh anode capacity. However, strong solvation of Li+ and slow interfacial ion transfer associated with conventional electrolytes limit their long-cycle and high-rate capabilities. Herein an electrolyte system based on fluoroalkyl ether 2,2,2-trifluoroethyl-1,1,2,3,3,3-hexafluoropropyl ether (THE) and ether electrolytes is designed to effectively upgrade the long-cycle and high-rate performances of LMBs. THE owns large adsorption energy with ether-based solvents, thus reducing Li+ interaction and solvation in ether electrolytes. With THE rich in fluoroalkyl groups adjacent to oxygen atoms, the electrolyte owns ultrahigh polarity, enabling solvation-free Li+ transfer with a substantially decreased energy barrier and ten times enhancement in Li+ transference at the electrolyte/anode interface. In addition, the uniform adsorption of fluorine-rich THE on the anode and subsequent LiF formation suppress dendrite formation and stabilize the solid electrolyte interphase layer. With the electrolyte, the lithium metal battery with a LiFePO4 cathode delivers unprecedented cyclic performances with only 0.0012% capacity loss per cycle over 5000 cycles at 10 C. Such enhancement is consistently observed for LMBs with other mainstream electrodes including LiCoO2 and LiNi0.5Mn0.3Co0.2O2, suggesting the generality of the electrolyte design for battery applications.
中文翻译:

高极性氟烷基醚电解质可实现高倍率锂金属电池的无溶剂化锂离子转移
锂金属电池(LMBs)由于具有超高的负极容量,在储能领域引起了广泛的关注。然而,与传统电解质相关的 Li +的强溶剂化和缓慢的界面离子转移限制了它们的长循环和高倍率能力。本文设计了一种基于氟烷基醚 2,2,2-三氟乙基-1,1,2,3,3,3-六氟丙基醚 (THE) 和醚电解质的电解质体系,以有效提升长循环和高倍率性能的 LMB。THE对醚类溶剂具有较大的吸附能,从而减少了醚电解质中的Li +相互作用和溶剂化。由于与氧原子相邻的富含氟烷基的TH,电解质具有超高极性,可实现无溶剂化的Li +在电解质/阳极界面处能量势垒显着降低,Li +迁移增强十倍。此外,富氟THE在阳极上的均匀吸附和随后的LiF形成抑制了枝晶的形成并稳定了固体电解质界面层。使用该电解质,具有 LiFePO 4正极的锂金属电池提供了前所未有的循环性能,在 10 C 下超过 5000 次循环后,每个循环的容量损失仅为 0.0012%。对于具有其他主流电极(包括 LiCoO 2和 LiNi 0.5 Mn )的 LMB 始终观察到这种增强0.3钴0.2氧2,表明电池应用的电解质设计的一般性。
更新日期:2021-12-19
中文翻译:

高极性氟烷基醚电解质可实现高倍率锂金属电池的无溶剂化锂离子转移
锂金属电池(LMBs)由于具有超高的负极容量,在储能领域引起了广泛的关注。然而,与传统电解质相关的 Li +的强溶剂化和缓慢的界面离子转移限制了它们的长循环和高倍率能力。本文设计了一种基于氟烷基醚 2,2,2-三氟乙基-1,1,2,3,3,3-六氟丙基醚 (THE) 和醚电解质的电解质体系,以有效提升长循环和高倍率性能的 LMB。THE对醚类溶剂具有较大的吸附能,从而减少了醚电解质中的Li +相互作用和溶剂化。由于与氧原子相邻的富含氟烷基的TH,电解质具有超高极性,可实现无溶剂化的Li +在电解质/阳极界面处能量势垒显着降低,Li +迁移增强十倍。此外,富氟THE在阳极上的均匀吸附和随后的LiF形成抑制了枝晶的形成并稳定了固体电解质界面层。使用该电解质,具有 LiFePO 4正极的锂金属电池提供了前所未有的循环性能,在 10 C 下超过 5000 次循环后,每个循环的容量损失仅为 0.0012%。对于具有其他主流电极(包括 LiCoO 2和 LiNi 0.5 Mn )的 LMB 始终观察到这种增强0.3钴0.2氧2,表明电池应用的电解质设计的一般性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号