Separation and Purification Technology ( IF 8.1 ) Pub Date : 2021-12-17 , DOI: 10.1016/j.seppur.2021.120307 Zhihao Guo 1, 2 , Sultan Ahmed Khoso 3 , Jinggang Wang 1, 2 , Chenyang Zhang 1, 2 , Zhiyong Gao 1, 2 , Wei Sun 1, 2 , Mengjie Tian 1, 2, 4 , Yuling Liu 5
|
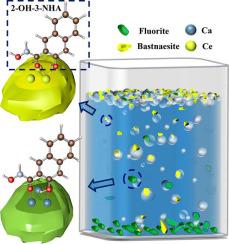
Bastnaesite ((Ce, La, Pr, Rb)CO3F) is an important source of light rare earth elements and is commonly associated with calcium (Ca)-containing minerals such as fluorite (CaF2), calcite (CaCO3) and dolomite ((Ca, Mg)(CO3)2). Froth flotation is an efficient method to separate bastnaesite and Ca-containing minerals. 2-hydroxy-3-naphthyl hydroxamic acid (2-OH-3-NHA) and 1-hydroxy-2-naphthyl hydroxamic acid (1-OH-2-NHA) are two widely used flotation collectors for bastnaesite and this manuscript investigates their performance and interaction mechanism in the flotation separation of bastnaesite/fluorite. The development of novel collectors must be based on the investigations of this manuscript. The flotation experimental results show the excellent selectivities of NHAs and the better selectivity of 1-OH-2-NHA than 2-OH-3-NHA. Zeta potential measurements suggest stronger adsorptions of NHAs on the bastnaesite surface than fluorite, which results in the excellent selectivities of NHAs. First-principles calculations demonstrate that naphthalene ring-bonded OH group of NHAs shows non-selective adsorption towards the surfaces of bastnaesite and fluorite. The better selectivity of 1-OH-2-NHA than 2-OH-3-NHA is explained by the lower reactivity of naphthalene ring-bonded OH group due to the stronger steric hindrance effect of naphthyl group towards this OH group.
中文翻译:

2-羟基-3-萘基异羟肟酸与1-羟基-2-萘基异羟肟酸在氟碳铈矿/萤石浮选分离中的相互作用机理:实验及第一性原理计算
氟碳铈矿 ((Ce, La, Pr, Rb)CO 3 F) 是轻稀土元素的重要来源,通常与含钙 (Ca) 矿物如萤石 (CaF 2 )、方解石 (CaCO 3 ) 和白云石 ((Ca, Mg)(CO 3 ) 2)。泡沫浮选是分离氟碳铈矿和含钙矿物的有效方法。2-羟基-3-萘基异羟肟酸 (2-OH-3-NHA) 和 1-羟基-2-萘基异羟肟酸 (1-OH-2-NHA) 是两种广泛使用的氟碳铈矿浮选捕收剂,本手稿研究了它们的氟碳铈矿/萤石浮选分离性能及相互作用机理[J]. 小说收藏家的发展,必须以对本手稿的调查为基础。浮选实验结果表明,NHAs具有优良的选择性,1-OH-2-NHA的选择性优于2-OH-3-NHA。Zeta 电位测量表明 NHA 在氟碳铈矿表面的吸附力强于萤石,这导致 NHA 具有优异的选择性。第一性原理计算表明,NHA 的萘环键合 OH 基团对氟碳铈矿和萤石表面显示出非选择性吸附。1-OH-2-NHA 的选择性比 2-OH-3-NHA 更好,这是由于萘基团对该 OH 基团的空间位阻效应更强,因此萘环键合的 OH 基团的反应性较低。

































 京公网安备 11010802027423号
京公网安备 11010802027423号