Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Porous Two-dimensional Iron-Cyano Nanosheets for High-rate Electrochemical Nitrate Reduction
ACS Nano ( IF 15.8 ) Pub Date : 2021-12-17 , DOI: 10.1021/acsnano.1c08814 Zhiwei Fang 1 , Zhaoyu Jin 2 , Sishuang Tang 1 , Panpan Li 1 , Ping Wu 3 , Guihua Yu 1
ACS Nano ( IF 15.8 ) Pub Date : 2021-12-17 , DOI: 10.1021/acsnano.1c08814 Zhiwei Fang 1 , Zhaoyu Jin 2 , Sishuang Tang 1 , Panpan Li 1 , Ping Wu 3 , Guihua Yu 1
Affiliation
|
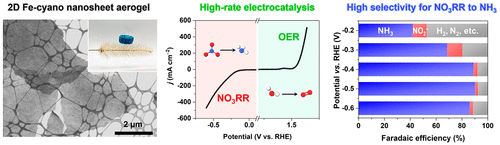
Ammonia (NH3) is an essential ingredient in agriculture and a promising source of clean energy as a hydrogen carrier. The current major method for ammonia production, however, is the Haber–Bosch process that leads to massive energy consumption and severe environmental issues. Compared with nitrogen (N2) reduction, electrochemical nitrate reduction reaction (NO3RR), with a higher NH3 yield rate and Faradaic efficiency, holds promise for efficient NH3 production under ambient conditions. To achieve efficient NO3RR, electrocatalysts should exhibit high selectivity and Faradaic efficiency with a high NH3 yield rate. In this work, we developed two-dimensional (2D) iron-based cyano-coordination polymer nanosheets (Fe-cyano NSs) following in situ electrochemical treatment for high-rate NO3RR. Owing to the strong adsorption of nitrate on Fe0 active sites generated via topotactic conversion and in situ electroreduction, 2D Fe-cyano electrocatalyst exhibits high catalytic activity with a yield rate of 42.1 mg h–1 mgcat–1 and a Faradaic efficiency of over 90% toward NH3 production at −0.5 V (vs reversible hydrogen electrode, RHE). Further electrochemical characterizations revealed that superhydrophilic surface and enhanced electrochemical surface area of the 2D porous nanostructures also contributed to the high-rate NO3RR activity. An electrolyzer toward NO3RR and oxygen evolution reaction (OER) in a two-electrode configuration is constructed based on 2D Fe-cyano, achieving an energy efficiency of 26.2%. This work provides an alternative methodology toward topotactic conversion of transition metal nanosheets for NO3RR and reveals the often-overlooked contribution of hydrophilicity of the catalysts for high-rate electrocatalysis.
中文翻译:

用于高倍率电化学硝酸盐还原的多孔二维铁氰基纳米片
氨(NH 3)是农业中必不可少的成分,也是作为氢载体的有前途的清洁能源来源。然而,目前氨生产的主要方法是 Haber-Bosch 工艺,该工艺会导致大量能源消耗和严重的环境问题。与氮(N 2)还原相比,电化学硝酸盐还原反应(NO 3 RR)具有更高的NH 3产率和法拉第效率,有望在环境条件下高效生产NH 3 。为了实现高效的 NO 3 RR,电催化剂应表现出高选择性和法拉第效率以及高 NH 3收益率。在这项工作中,我们开发了二维 (2D) 铁基氰基配位聚合物纳米片 (Fe-cyano NSs) ,并在原位电化学处理后对高倍率 NO 3 RR 进行了处理。由于通过拓扑转化和原位电还原产生的 Fe 0活性位点上强烈吸附硝酸盐,二维 Fe-氰基电催化剂表现出高催化活性,产率为 42.1 mg h -1 mg cat -1,法拉第效率超过90% 用于在 -0.5 V 时产生NH 3 (与可逆氢电极,RHE)。进一步的电化学表征表明,二维多孔纳米结构的超亲水表面和增强的电化学表面积也有助于高速 NO 3 RR 活性。基于二维铁氰基构建了一种双电极配置的NO 3 RR 和析氧反应 (OER) 电解槽,能效达到 26.2%。这项工作提供了一种过渡金属纳米片拓扑转化用于 NO 3 RR 的替代方法,并揭示了催化剂的亲水性在高速电催化中经常被忽视的贡献。
更新日期:2022-01-25
中文翻译:

用于高倍率电化学硝酸盐还原的多孔二维铁氰基纳米片
氨(NH 3)是农业中必不可少的成分,也是作为氢载体的有前途的清洁能源来源。然而,目前氨生产的主要方法是 Haber-Bosch 工艺,该工艺会导致大量能源消耗和严重的环境问题。与氮(N 2)还原相比,电化学硝酸盐还原反应(NO 3 RR)具有更高的NH 3产率和法拉第效率,有望在环境条件下高效生产NH 3 。为了实现高效的 NO 3 RR,电催化剂应表现出高选择性和法拉第效率以及高 NH 3收益率。在这项工作中,我们开发了二维 (2D) 铁基氰基配位聚合物纳米片 (Fe-cyano NSs) ,并在原位电化学处理后对高倍率 NO 3 RR 进行了处理。由于通过拓扑转化和原位电还原产生的 Fe 0活性位点上强烈吸附硝酸盐,二维 Fe-氰基电催化剂表现出高催化活性,产率为 42.1 mg h -1 mg cat -1,法拉第效率超过90% 用于在 -0.5 V 时产生NH 3 (与可逆氢电极,RHE)。进一步的电化学表征表明,二维多孔纳米结构的超亲水表面和增强的电化学表面积也有助于高速 NO 3 RR 活性。基于二维铁氰基构建了一种双电极配置的NO 3 RR 和析氧反应 (OER) 电解槽,能效达到 26.2%。这项工作提供了一种过渡金属纳米片拓扑转化用于 NO 3 RR 的替代方法,并揭示了催化剂的亲水性在高速电催化中经常被忽视的贡献。































 京公网安备 11010802027423号
京公网安备 11010802027423号