Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2021-12-17 , DOI: 10.1016/j.jcis.2021.12.085
Yu-Jen Shih , Pei-Ying Lin , Zhi-Lun Wu
|
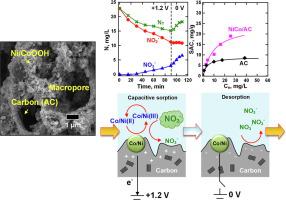
The composite electrode of NiCo oxide supported by porous carbon was synthesized for nitrite oxidation and nitrate electro-sorption. The crystal structure and chemical state of the Co and Ni oxyhydroxides which were precipitated on loofah-derived activated carbon (AC) using hypochlorite were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), X-ray photoelectron spectroscopy (XPS), and BET surface area. The voltammetry showed that the redox couple of Co(II)/Co(III) and Ni(II)/Ni(III) as the mediator catalytically transferred the electrons of NO2–/NO3–; the Ni site had a relatively high transfer coefficient and diffusive current, while the Co site was better in the capacitive removal of the nitrite and nitrate compounds. A batch electrolysis of nitrite ions was operated under constant anodic potential mode (0 to + 1.5 V vs. Ag/AgCl) to assess the performance of the composite electrodes. The adsorption capacity of NiCo/AC (Ni = 5% and Co = 5% on AC by weight) was 23.5 mg-N g−1, which was twice that of AC substrate (7.5 mg-N g−1), based on a multilayer adsorption model. The steady-state kinetics of the consecutive reaction were derived to determine the rate steps of the electrochemical oxidation of NO2– and adsorption of NO3–.
中文翻译:

使用介孔碳负载的纳米片状钴和羟基氧化镍催化氧化和去离子亚硝酸根和硝酸根离子
合成了多孔碳负载的NiCo氧化物复合电极,用于亚硝酸盐氧化和硝酸盐电吸附。利用次氯酸盐在丝瓜衍生的活性炭(AC)上沉淀出的Co和Ni羟基氧化物的晶体结构和化学状态通过X射线衍射(XRD)、扫描电子显微镜(SEM)、X射线光电子能谱( XPS) 和 BET 表面积。伏安法表明,Co(II)/Co(III)和Ni(II)/Ni(III)的氧化还原电对作为介体催化转移了NO 2 - /NO 3 -的电子。; Ni位具有相对较高的传递系数和扩散电流,而Co位在亚硝酸盐和硝酸盐化合物的电容去除方面更好。在恒定阳极电位模式(0 至 + 1.5 V vs. Ag/AgCl)下进行亚硝酸根离子的批量电解,以评估复合电极的性能。NiCo/AC (Ni = 5% and Co = 5% on AC) 的吸附容量为 23.5 mg-N g -1,是 AC 底物 (7.5 mg-N g -1 ) 的两倍,基于多层吸附模型。推导出连续反应的稳态动力学以确定电化学氧化 NO 2 -和吸附 NO 3 -的速率步骤。

































 京公网安备 11010802027423号
京公网安备 11010802027423号