当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Electrosynthesis of Urea from Carbon Dioxide and Nitric Oxide
ACS Energy Letters ( IF 19.3 ) Pub Date : 2021-12-15 , DOI: 10.1021/acsenergylett.1c02471 Yanmei Huang 1 , Rong Yang 1 , Changhong Wang 1 , Nannan Meng 1 , Yanmei Shi 1 , Yifu Yu 1 , Bin Zhang 1, 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2021-12-15 , DOI: 10.1021/acsenergylett.1c02471 Yanmei Huang 1 , Rong Yang 1 , Changhong Wang 1 , Nannan Meng 1 , Yanmei Shi 1 , Yifu Yu 1 , Bin Zhang 1, 2
Affiliation
|
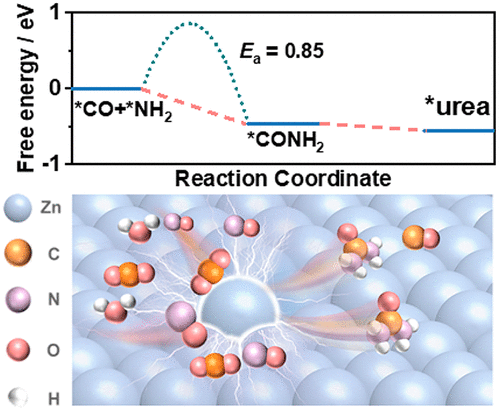
Electrochemical synthesis of urea provides a sustainable strategy that can be easily incorporated into currently distributed renewable energy systems. The main challenge that hindered the advancement of this technique lies in developing advanced electrocatalytic processes to utilize abundant and low-cost inorganic carbon and nitrogen sources for highly productive urea generation. Herein, we report an electrocatalytic reaction that converts carbon dioxide (CO2) and nitric oxide (NO) into urea, with water as the hydrogen source, under ambient conditions. The yield rate and Faradaic efficiency of urea reach 15.13 mmol g–1 h–1 and 11.26% at a current density of 40 mA cm–2 under optimized conditions. The critical intermediates of *CO and *NH2 for urea generation are obtained via the co-reduction of CO2 and NO and then continuously interconnect to form the C–N bond. A preliminary techno-economic study is performed to discuss the practical application potential of this strategy for urea production.
中文翻译:

二氧化碳和一氧化氮直接电合成尿素
尿素的电化学合成提供了一种可持续的策略,可以很容易地融入当前分布式的可再生能源系统中。阻碍该技术进步的主要挑战在于开发先进的电催化工艺,以利用丰富且低成本的无机碳和氮源来生产高产尿素。在此,我们报告了一种电催化反应,该反应在环境条件下以水为氢源,将二氧化碳 (CO 2 ) 和一氧化氮 (NO) 转化为尿素。在电流密度为 40 mA cm -2时,尿素的产率和法拉第效率分别达到 15.13 mmol g -1 h -1和 11.26%在优化的条件下。生成尿素的*CO和*NH 2的关键中间体是通过CO 2和NO的共还原得到的,然后不断互连形成C-N键。进行了初步的技术经济研究,以讨论该策略在尿素生产中的实际应用潜力。
更新日期:2022-01-14
中文翻译:

二氧化碳和一氧化氮直接电合成尿素
尿素的电化学合成提供了一种可持续的策略,可以很容易地融入当前分布式的可再生能源系统中。阻碍该技术进步的主要挑战在于开发先进的电催化工艺,以利用丰富且低成本的无机碳和氮源来生产高产尿素。在此,我们报告了一种电催化反应,该反应在环境条件下以水为氢源,将二氧化碳 (CO 2 ) 和一氧化氮 (NO) 转化为尿素。在电流密度为 40 mA cm -2时,尿素的产率和法拉第效率分别达到 15.13 mmol g -1 h -1和 11.26%在优化的条件下。生成尿素的*CO和*NH 2的关键中间体是通过CO 2和NO的共还原得到的,然后不断互连形成C-N键。进行了初步的技术经济研究,以讨论该策略在尿素生产中的实际应用潜力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号