当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Synthesis of 2,3-Dihydrobenzo[b]oxepines via Asymmetric Oxetane Opening by Internal Carbon Nucleophiles
Organic Letters ( IF 4.9 ) Pub Date : 2021-12-16 , DOI: 10.1021/acs.orglett.1c03852 Tianyu Zhang 1 , Han Zhuang 1 , Luning Tang 1 , Zhengyu Han 1 , Wengang Guo 1 , Hai Huang 1 , Jianwei Sun 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2021-12-16 , DOI: 10.1021/acs.orglett.1c03852 Tianyu Zhang 1 , Han Zhuang 1 , Luning Tang 1 , Zhengyu Han 1 , Wengang Guo 1 , Hai Huang 1 , Jianwei Sun 1, 2
Affiliation
|
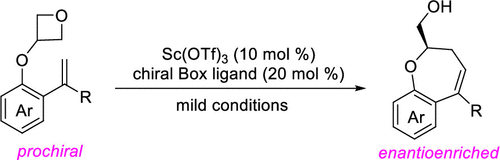
An intramolecular C–C formation process based on catalytic asymmetric oxetane opening by carbon nucleophiles has been developed, which provides rapid access to a range of valuable enantioenriched 2,3-dihydrobenzo[b]oxepines. With the combination of Sc(OTf)3 and a Box ligand, good chemical efficiency and enantioselectivity were achieved under mild conditions. The products are also useful precursors to other valuable structures, such as the bicyclo[3.2.2]nonane derivatives.
中文翻译:

内碳亲核试剂不对称氧杂环丁烷开环催化对映选择性合成2,3-二氢苯并[b]氧杂环丁烷
已经开发出一种基于碳亲核试剂催化不对称氧杂环丁烷开环的分子内 C-C 形成过程,它可以快速获得一系列有价值的对映体富集的 2,3-二氢苯并[ b ]氧杂环丁烷。Sc(OTf) 3和Box配体的结合,在温和条件下实现了良好的化学效率和对映选择性。该产品也是其他有价值结构的有用前体,例如双环[3.2.2]壬烷衍生物。
更新日期:2022-01-14
中文翻译:

内碳亲核试剂不对称氧杂环丁烷开环催化对映选择性合成2,3-二氢苯并[b]氧杂环丁烷
已经开发出一种基于碳亲核试剂催化不对称氧杂环丁烷开环的分子内 C-C 形成过程,它可以快速获得一系列有价值的对映体富集的 2,3-二氢苯并[ b ]氧杂环丁烷。Sc(OTf) 3和Box配体的结合,在温和条件下实现了良好的化学效率和对映选择性。该产品也是其他有价值结构的有用前体,例如双环[3.2.2]壬烷衍生物。








































 京公网安备 11010802027423号
京公网安备 11010802027423号