当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Violations. How Nature Circumvents the Woodward–Hoffmann Rules and Promotes the Forbidden Conrotatory 4n + 2 Electron Electrocyclization of Prinzbach’s Vinylogous Sesquifulvalene
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-16 , DOI: 10.1021/jacs.1c11058 Garrett A Kukier 1 , Aneta Turlik 1 , Xiao-Song Xue 1 , K N Houk 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-12-16 , DOI: 10.1021/jacs.1c11058 Garrett A Kukier 1 , Aneta Turlik 1 , Xiao-Song Xue 1 , K N Houk 1
Affiliation
|
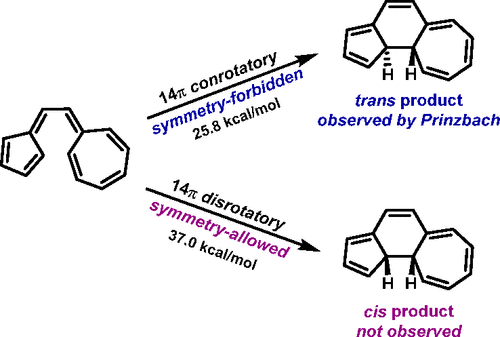
Woodward and Hoffmann, in their treatise on orbital symmetry in 1969, stated “Violations. There are none!” Prinzbach reported in 1978 that the electrocyclization of vinylogous sesquifulvalene occurs exclusively through the Woodward–Hoffmann orbital-symmetry-forbidden 14π-electron conrotatory pathway, despite the availability of a variety of orbital-symmetry-allowed processes. Prinzbach later demonstrated that an 18π-electron homologue exhibits the same forbidden behavior. And yet, the analogous vinylogous pentafulvalene and heptafulvalene both follow the orbital symmetry rules, each proceeding through its allowed conrotatory 12π and 16π process, respectively. We report the investigation of these reactions with ωB97X-D DFT. The physical origins of the flagrant Prinzbach violations of the Woodward–Hoffmann orbital symmetry selection rules have now been elucidated by these calculations in conjunction with extensive analyses and comparisons to electrocyclizations that obey the Woodward–Hoffmann rules. This remarkable reversal of the Rules (the 14π-electron-forbidden process is found to be 11 kcal/mol more energetically facile than the allowed process) occurs due to the high degree of polarization of this hydrocarbon, such that conrotatory electrocyclization of vinylogous sesquifulvalene behaves like a cyclopentadienide combining with a tropylium. These results are compared to other forbidden pericyclic processes driven by steric constraints and strain release or by diradical character of the reactants that facilitates the formation of diradical transition states for symmetry-forbidden reactions. We predict how strong donor–acceptor substitution can modify nodal properties to level the difference between allowed and forbidden electrocyclic reaction barriers, and we provide computational predictions of two such cases.
中文翻译:

违规行为。大自然如何规避伍德沃德-霍夫曼规则并促进 Prinzbach 乙烯基倍半富瓦烯的禁止旋转 4n + 2 电子电环化
伍德沃德和霍夫曼在 1969 年关于轨道对称性的论文中指出:“违反。没有了!” Prinzbach 在 1978 年报道,尽管存在多种轨道对称允许的过程,但乙烯基倍半富瓦烯的电环化仅通过 Woodward-Hoffmann 轨道对称禁止的 14π-电子旋转途径发生。Prinzbach 后来证明 18π 电子同系物表现出相同的禁止行为。然而,类似的插烯五富瓦烯和七富瓦烯都遵循轨道对称规则,分别通过其允许的自转 12π 和 16π 过程。我们报告了使用 ωB97X-D DFT 对这些反应的研究。这些计算以及与遵循伍德沃德-霍夫曼规则的电环化的广泛分析和比较,现已阐明了公然违反伍德沃德-霍夫曼轨道对称性选择规则的普林茨巴赫的物理起源。规则的这种显着逆转(发现 14π 电子禁止过程比允许的过程在能量上更容易 11 kcal/mol)是由于这种烃的高度极化而发生的,因此乙烯基倍半富瓦烯的旋转电环化表现出就像与 tropylium 结合的环戊二烯。将这些结果与其他由空间约束和应变释放或反应物的双自由基特性驱动的禁止的周环过程进行比较,这有助于形成对称禁止反应的双自由基过渡态。我们预测了强供体 - 受体替代可以如何改变节点特性以平衡允许和禁止的电循环反应势垒之间的差异,并且我们提供了两种此类情况的计算预测。
更新日期:2021-12-29
中文翻译:

违规行为。大自然如何规避伍德沃德-霍夫曼规则并促进 Prinzbach 乙烯基倍半富瓦烯的禁止旋转 4n + 2 电子电环化
伍德沃德和霍夫曼在 1969 年关于轨道对称性的论文中指出:“违反。没有了!” Prinzbach 在 1978 年报道,尽管存在多种轨道对称允许的过程,但乙烯基倍半富瓦烯的电环化仅通过 Woodward-Hoffmann 轨道对称禁止的 14π-电子旋转途径发生。Prinzbach 后来证明 18π 电子同系物表现出相同的禁止行为。然而,类似的插烯五富瓦烯和七富瓦烯都遵循轨道对称规则,分别通过其允许的自转 12π 和 16π 过程。我们报告了使用 ωB97X-D DFT 对这些反应的研究。这些计算以及与遵循伍德沃德-霍夫曼规则的电环化的广泛分析和比较,现已阐明了公然违反伍德沃德-霍夫曼轨道对称性选择规则的普林茨巴赫的物理起源。规则的这种显着逆转(发现 14π 电子禁止过程比允许的过程在能量上更容易 11 kcal/mol)是由于这种烃的高度极化而发生的,因此乙烯基倍半富瓦烯的旋转电环化表现出就像与 tropylium 结合的环戊二烯。将这些结果与其他由空间约束和应变释放或反应物的双自由基特性驱动的禁止的周环过程进行比较,这有助于形成对称禁止反应的双自由基过渡态。我们预测了强供体 - 受体替代可以如何改变节点特性以平衡允许和禁止的电循环反应势垒之间的差异,并且我们提供了两种此类情况的计算预测。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号