当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosting Cancer Immunotherapy via the Convenient A2AR Inhibition Using a Tunable Nanocatalyst with Light-Enhanced Activity
Advanced Materials ( IF 27.4 ) Pub Date : 2021-12-15 , DOI: 10.1002/adma.202106967 Wenqian Yu 1 , Junlin Sun 1 , Xiuyuan Wang 1 , Shuyi Yu 1 , Mingzhu Yan 1 , Fuan Wang 1 , Xiaoqing Liu 1
Advanced Materials ( IF 27.4 ) Pub Date : 2021-12-15 , DOI: 10.1002/adma.202106967 Wenqian Yu 1 , Junlin Sun 1 , Xiuyuan Wang 1 , Shuyi Yu 1 , Mingzhu Yan 1 , Fuan Wang 1 , Xiaoqing Liu 1
Affiliation
|
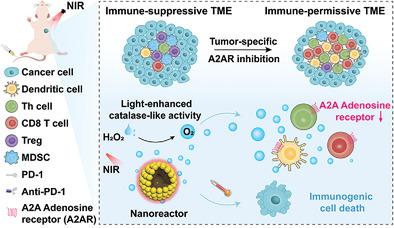
Blockade of A2A adenosine receptors (A2AR)-adenosinergic signaling shows high potency to mobilize antitumor immunity for its in-depth involvement in immune regulation of nearly all immune cells. Available A2AR inhibition strategies are mainly based on small molecules or proteins inhibitors, yet are limited by the non-specific operation as well as the off-target toxicity. Herein, the first effort to design a convenient tumor-specific A2AR inhibition strategy to improve antitumor immune responses via the spatiotemporally controlled oxygen supply by virtue of a versatile photo-modulated nanoreactor is reported on. This nanoreactor, consisting of a catalase-mimicking shell (Pt nanocatalyst) and a photothermal core (polydopamine), is rationally designed for achieving the near-infrared radiation (NIR)-guided/accelerated oxygen supplementation on tumor site, and for relieving the A2AR-mediated immunosuppression without toxicity concern. Meanwhile, the NIR light could also mediate the direct photothermal ablation of tumor, and elicit immunogenic cell deaths to boost antitumor immunity. In a poorly immunogenic breast cancer model, the intravenous injection of the nanoreactor leads to the improved immune response with an increased animal survival rate, and achieves the long-term immunological memory effect against tumor recurrence as well as rechallenge. This convenient nanoreactor-stimulated A2AR inhibition approach provides a versatile promising paradigm for improving these existing immunotherapies.
中文翻译:

使用具有光增强活性的可调纳米催化剂通过方便的 A2AR 抑制促进癌症免疫治疗
阻断 A2A 腺苷受体 (A2AR)-腺苷能信号传导显示出调动抗肿瘤免疫的高效力,因为它深入参与了几乎所有免疫细胞的免疫调节。可用的 A2AR 抑制策略主要基于小分子或蛋白质抑制剂,但受限于非特异性操作以及脱靶毒性。在此,首次尝试设计一种方便的肿瘤特异性 A2AR 抑制策略,以借助多功能光调制纳米反应器通过时空控制的氧气供应来改善抗肿瘤免疫反应。该纳米反应器由模拟过氧化氢酶的壳(Pt 纳米催化剂)和光热核(聚多巴胺)组成,设计合理,可在肿瘤部位实现近红外辐射 (NIR) 引导/加速补氧,并缓解 A2AR 介导的免疫抑制而无毒性问题。同时,近红外光还可以介导肿瘤的直接光热消融,引发免疫原性细胞死亡,从而增强抗肿瘤免疫力。在免疫原性较差的乳腺癌模型中,静脉注射纳米反应器可改善免疫反应,提高动物存活率,并实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。并用于缓解 A2AR 介导的免疫抑制而无毒性问题。同时,近红外光还可以介导肿瘤的直接光热消融,引发免疫原性细胞死亡,从而增强抗肿瘤免疫力。在免疫原性较差的乳腺癌模型中,静脉注射纳米反应器可改善免疫反应,提高动物存活率,并实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。并用于缓解 A2AR 介导的免疫抑制而无毒性问题。同时,近红外光还可以介导肿瘤的直接光热消融,引发免疫原性细胞死亡,从而增强抗肿瘤免疫力。在免疫原性较差的乳腺癌模型中,静脉注射纳米反应器可改善免疫反应,提高动物存活率,并实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。纳米反应器的静脉注射可改善免疫反应,提高动物存活率,实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。纳米反应器的静脉注射可改善免疫反应,提高动物存活率,实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。
更新日期:2021-12-15
中文翻译:

使用具有光增强活性的可调纳米催化剂通过方便的 A2AR 抑制促进癌症免疫治疗
阻断 A2A 腺苷受体 (A2AR)-腺苷能信号传导显示出调动抗肿瘤免疫的高效力,因为它深入参与了几乎所有免疫细胞的免疫调节。可用的 A2AR 抑制策略主要基于小分子或蛋白质抑制剂,但受限于非特异性操作以及脱靶毒性。在此,首次尝试设计一种方便的肿瘤特异性 A2AR 抑制策略,以借助多功能光调制纳米反应器通过时空控制的氧气供应来改善抗肿瘤免疫反应。该纳米反应器由模拟过氧化氢酶的壳(Pt 纳米催化剂)和光热核(聚多巴胺)组成,设计合理,可在肿瘤部位实现近红外辐射 (NIR) 引导/加速补氧,并缓解 A2AR 介导的免疫抑制而无毒性问题。同时,近红外光还可以介导肿瘤的直接光热消融,引发免疫原性细胞死亡,从而增强抗肿瘤免疫力。在免疫原性较差的乳腺癌模型中,静脉注射纳米反应器可改善免疫反应,提高动物存活率,并实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。并用于缓解 A2AR 介导的免疫抑制而无毒性问题。同时,近红外光还可以介导肿瘤的直接光热消融,引发免疫原性细胞死亡,从而增强抗肿瘤免疫力。在免疫原性较差的乳腺癌模型中,静脉注射纳米反应器可改善免疫反应,提高动物存活率,并实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。并用于缓解 A2AR 介导的免疫抑制而无毒性问题。同时,近红外光还可以介导肿瘤的直接光热消融,引发免疫原性细胞死亡,从而增强抗肿瘤免疫力。在免疫原性较差的乳腺癌模型中,静脉注射纳米反应器可改善免疫反应,提高动物存活率,并实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。纳米反应器的静脉注射可改善免疫反应,提高动物存活率,实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。纳米反应器的静脉注射可改善免疫反应,提高动物存活率,实现对肿瘤复发和再攻击的长期免疫记忆效应。这种方便的纳米反应器刺激的 A2AR 抑制方法为改进这些现有的免疫疗法提供了一种通用的有前途的范例。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号