Science of the Total Environment ( IF 8.2 ) Pub Date : 2021-12-15 , DOI: 10.1016/j.scitotenv.2021.152242 Yun Luo 1 , Baoqin Zhang 2 , Ningbo Geng 2 , Shuai Sun 1 , Xiaoyao Song 2 , Jiping Chen 2 , Haijun Zhang 2
|
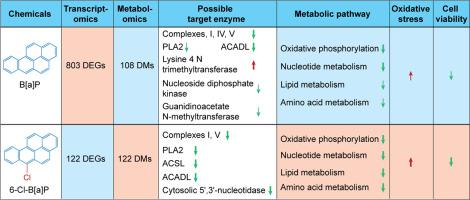
The toxicological information of chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs), as derivatives of PAHs, is still relatively lacking. In this study, a combination of transcriptomics and metabolomics approach was adopted to explore the changes in toxicity to human L02 hepatocytes after chlorination of benzo[a]pyrene (B[a]P) at 6 position. In general, 6-Cl-B[a]P produced a stronger toxicity to human hepatic cells than did parent B[a]P. When exposure concentrations were 5 and 50 nM, 6-Cl-B[a]P caused a weaker transcriptomic perturbation relative to B[a]P, whereas a stronger metabolomic perturbation, a stronger oxidative stress and a stronger inhibition effect on cell viability were caused by 6-Cl-B[a]P than did parent B[a]P. Pathway enrichment analysis indicated that 6-Cl-B[a]P produced a more widely perturbation to metabolic pathways than did B[a]P. Although they both significantly impaired the function of mitochondrial electron transport chain (ETC), the exact mechanism is different. B[a]P suppressed the expression of 20 genes regulating mitochondrial ETC mainly via AhR activation. However, 6-Cl-B[a]P produced a stronger inhibition on the activities of complexes I and V than did B[a]P. Meanwhile, 6-Cl-B[a]P also exhibited a stronger inhibition effect on mitochondrial β oxidation of fatty acid. Furthermore, 6-Cl-B[a]P and B[a]P both significantly disturbed the nucleotide metabolism, glycerophospholipid metabolism and amino acid metabolism in L02 cells.
中文翻译:

转录组学和代谢组学分析提供了对苯并[a]芘和6-氯苯并[a]芘对人肝细胞毒性差异的见解
作为多环芳烃衍生物的氯化多环芳烃(Cl-PAHs)的毒理学信息仍然相对缺乏。本研究采用转录组学和代谢组学相结合的方法,探讨苯并[ a ]芘(B[ a ]P)6位氯化后对人L02肝细胞毒性的变化。一般来说,6-Cl-B[ a ]P对人肝细胞的毒性比母体B[ a ]P更强。当暴露浓度为 5 和 50 nM 时,6-Cl-B[ a]P 相对于 B[a ] P 引起较弱的转录组扰动]P,而与亲本 B[ a ]P 相比,6-Cl-B[ a ]P引起更强的代谢组学扰动、更强的氧化应激和对细胞活力的更强抑制作用。通路富集分析表明,6-Cl-B[ a ]P 比 B[ a ]P对代谢途径产生更广泛的扰动。虽然它们都显着损害了线粒体电子传递链(ETC)的功能,但确切的机制是不同的。B[ a ]P主要通过AhR激活抑制20个调节线粒体ETC基因的表达。然而,6-Cl-B[ a ]P 对配合物 I 和 V 的抑制作用比 B[a]P 更强。]P。同时,6-Cl-B[ a ]P对脂肪酸线粒体β氧化也表现出更强的抑制作用。此外,6-Cl-B[ a ]P和B[ a ]P均显着干扰L02细胞的核苷酸代谢、甘油磷脂代谢和氨基酸代谢。






























 京公网安备 11010802027423号
京公网安备 11010802027423号