当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituent-Enhanced Intermolecular Catalytic Ene-yne Metathesis for Efficient 1,3-Diene Synthesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-12-14 , DOI: 10.1021/acscatal.1c04074 Kevin Basemann 1 , Bradley M. Schmidt 1 , Aaron D. Sadow 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-12-14 , DOI: 10.1021/acscatal.1c04074 Kevin Basemann 1 , Bradley M. Schmidt 1 , Aaron D. Sadow 1
Affiliation
|
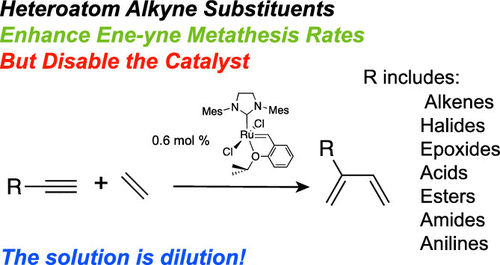
The appropriate reaction conditions for ruthenium-catalyzed ene-yne metathesis are substantially affected by the substituents and functional groups on the terminal alkyne reactant. We have identified that two distinct methods, utilizing a single precatalyst IMes(Cl)2Ru(═CHC6H4OiPr) (1; IMes = 1,3-dimesitylimidazol-2-ylidene), are needed to achieve high turnovers and good yields in ene-yne metathesis reactions of heteroatom-free or functionalized terminal alkynes and ethylene. The wide-ranging yields of 1,3-dienes from ene-yne metathesis of a series of terminal alkynes under a single set of benchmark conditions, namely, 3 mol % of 1, toluene, 75 °C, and 20 bar C2H4, identified groups of highly, moderately, or poorly performing alkynes. Studies of the effects of reaction conditions on yields, turnovers, and effective rates in the reactions of alkynes from these groups lead to distinct optimized approaches for efficacious syntheses. Quantitative yields are obtained using 0.6 mol % of 1, one fifth of the benchmark loading, in preparative-scale experiments under conventional conditions in which the highly efficient alkyne and 1 are mixed, followed by pressurization with ethylene. In contrast, the metered addition method, in which a moderately or poorly performing alkyne and a solution of 1 are slowly and separately added to an ethylene-pressurized reaction vessel, can improve turnovers up to 1500% to access 2-substituted-1,3-diene-containing carboxylic acids, carbonyls, amines, epoxides, or halides in a single step. Moreover, the increased effective rates of reactions for functionalized alkynes under dilute conditions, and comparisons with heteroatom-free alkyne conversions, suggest that the former reacts more rapidly than the latter in ene-yne metathesis, but their transformations are also more sensitive to catalyst deactivation.
中文翻译:

取代基增强分子间催化烯-炔复分解用于高效合成 1,3-二烯
钌催化的烯-炔复分解的合适反应条件主要受末端炔烃反应物上的取代基和官能团的影响。我们已经确定需要两种不同的方法,利用单一的预催化剂 IMes(Cl) 2 Ru(=CHC 6 H 4 O i Pr) ( 1 ; IMes = 1,3-dimesitylimidazol-2-ylidene) 来实现高周转率并且在无杂原子或官能化末端炔烃和乙烯的烯-炔复分解反应中具有良好的产率。在一组基准条件(即 3 mol% 的1、甲苯、75 °C 和 20 bar C 2H 4,识别出高、中或差表现的炔烃组。研究反应条件对来自这些基团的炔烃反应中的产率、周转率和有效速率的影响,导致了有效合成的不同优化方法。在常规条件下的制备规模实验中,使用 0.6 mol% 的1(基准负载量的五分之一)获得定量产率,其中高效炔烃和1混合,然后用乙烯加压。与此相反,在计量加入的方法,其中中度或效果不佳的炔和的溶液1缓慢且单独地添加到乙烯加压反应容器中,可以提高高达 1500% 的周转率,以在一个步骤中获得含 2-取代-1,3-二烯的羧酸、羰基、胺、环氧化物或卤化物。此外,在稀释条件下官能化炔烃的有效反应速率增加,并与无杂原子的炔烃转化率进行比较,表明前者在烯-炔复分解中的反应比后者更快,但它们的转化对催化剂失活也更敏感.
更新日期:2022-01-07
中文翻译:

取代基增强分子间催化烯-炔复分解用于高效合成 1,3-二烯
钌催化的烯-炔复分解的合适反应条件主要受末端炔烃反应物上的取代基和官能团的影响。我们已经确定需要两种不同的方法,利用单一的预催化剂 IMes(Cl) 2 Ru(=CHC 6 H 4 O i Pr) ( 1 ; IMes = 1,3-dimesitylimidazol-2-ylidene) 来实现高周转率并且在无杂原子或官能化末端炔烃和乙烯的烯-炔复分解反应中具有良好的产率。在一组基准条件(即 3 mol% 的1、甲苯、75 °C 和 20 bar C 2H 4,识别出高、中或差表现的炔烃组。研究反应条件对来自这些基团的炔烃反应中的产率、周转率和有效速率的影响,导致了有效合成的不同优化方法。在常规条件下的制备规模实验中,使用 0.6 mol% 的1(基准负载量的五分之一)获得定量产率,其中高效炔烃和1混合,然后用乙烯加压。与此相反,在计量加入的方法,其中中度或效果不佳的炔和的溶液1缓慢且单独地添加到乙烯加压反应容器中,可以提高高达 1500% 的周转率,以在一个步骤中获得含 2-取代-1,3-二烯的羧酸、羰基、胺、环氧化物或卤化物。此外,在稀释条件下官能化炔烃的有效反应速率增加,并与无杂原子的炔烃转化率进行比较,表明前者在烯-炔复分解中的反应比后者更快,但它们的转化对催化剂失活也更敏感.

































 京公网安备 11010802027423号
京公网安备 11010802027423号