当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical study and application of 2-phenyl-1,3,4-thiadiazole derivatives with optical and inhibitory activity against SHP1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-12-02 , DOI: 10.1039/d1cp04268h Chun Zhang 1 , Yi-Tao Sun 1 , Li-Xin Gao 1, 2 , Bo Feng 1, 2 , Xue Yan 1 , Xue-Hui Guo 3 , Ai-Min Ren 3 , Yu-Bo Zhou 2, 4 , Jia Li 2, 4 , Wen-Long Wang 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-12-02 , DOI: 10.1039/d1cp04268h Chun Zhang 1 , Yi-Tao Sun 1 , Li-Xin Gao 1, 2 , Bo Feng 1, 2 , Xue Yan 1 , Xue-Hui Guo 3 , Ai-Min Ren 3 , Yu-Bo Zhou 2, 4 , Jia Li 2, 4 , Wen-Long Wang 1
Affiliation
|
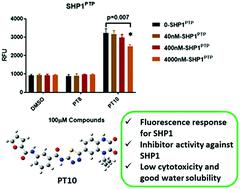
Src homology-2 domain-containing protein tyrosine phosphatase 1 (SHP1) is mainly restricted to hematopoietic and epithelial cells and widely accepted as a convergent node for oncogenic cell-signaling cascades. The development of efficient methods for rapidly tracing and inhibiting the SHP1 activity in complex biological systems is of considerable significance for advancing the integration of diagnosis and treatment of the related disease. With this aim, we designed and synthesized five 2-phenyl-1,3,4-thiadiazole derivatives (PT2, PT5, PT8, PT9 and PT10) here based on the reported SHP1 inhibitors (PT1, PT3, PT4, PT6 and PT7). The photophysical properties and inhibitory activities of these 2-phenyl-1,3,4-thiadiazole derivatives (PT1–PT10) against SHP1 were thoroughly studied from the theoretical simulation and experimental application aspects. The representative compound PT10 exhibited a larger quantum yield than the other molecules because of the smaller geometric relaxation and reorganization energy of the excited state, which was consistent with the results from the fluorescence experiments in organic solvents. In addition, PT10 showed a selective fluorescence response for SHP1 activity and low cytotoxicity in HeLa cells. Lastly, it indicated the potential application in two-photon cell fluorescence imaging in the future according to the calculated excellent two-photon absorption properties. In this contribution, firstly, we offered the fluorescent and activated molecule PT10 against SHP1, which achieved the integration of visualization and inhibitory activity of SHP1 preliminarily at the enzyme molecular level.
中文翻译:

对SHP1具有光学和抑制活性的2-苯基-1,3,4-噻二唑衍生物的理论研究与应用
含有 Src 同源 2 结构域的蛋白酪氨酸磷酸酶 1 (SHP1) 主要限于造血细胞和上皮细胞,并被广泛接受为致癌细胞信号级联的会聚节点。开发有效的快速追踪和抑制复杂生物系统中SHP1活性的方法,对于推进相关疾病的诊治一体化具有重要意义。为此,我们在此基于已报道的 SHP1 抑制剂(PT1、PT3、PT4、PT6 和 PT7)设计并合成了五种 2-苯基-1,3,4-噻二唑衍生物(PT2、PT5、PT8、PT9 和 PT10)。 . 从理论模拟和实验应用两方面深入研究了这些2-苯基-1,3,4-噻二唑衍生物(PT1-PT10)对SHP1的光物理性质和抑制活性。由于激发态的几何弛豫和重组能较小,代表性化合物PT10比其他分子表现出更大的量子产率,这与有机溶剂中荧光实验的结果一致。此外,PT10 对 HeLa 细胞中的 SHP1 活性和低细胞毒性显示出选择性荧光反应。最后,根据计算出的优异的双光子吸收特性,表明了未来在双光子细胞荧光成像中的潜在应用。在这项贡献中,我们首先提供了针对SHP1的荧光活化分子PT10,初步实现了SHP1在酶分子水平上的可视化和抑制活性的整合。
更新日期:2021-12-15
中文翻译:

对SHP1具有光学和抑制活性的2-苯基-1,3,4-噻二唑衍生物的理论研究与应用
含有 Src 同源 2 结构域的蛋白酪氨酸磷酸酶 1 (SHP1) 主要限于造血细胞和上皮细胞,并被广泛接受为致癌细胞信号级联的会聚节点。开发有效的快速追踪和抑制复杂生物系统中SHP1活性的方法,对于推进相关疾病的诊治一体化具有重要意义。为此,我们在此基于已报道的 SHP1 抑制剂(PT1、PT3、PT4、PT6 和 PT7)设计并合成了五种 2-苯基-1,3,4-噻二唑衍生物(PT2、PT5、PT8、PT9 和 PT10)。 . 从理论模拟和实验应用两方面深入研究了这些2-苯基-1,3,4-噻二唑衍生物(PT1-PT10)对SHP1的光物理性质和抑制活性。由于激发态的几何弛豫和重组能较小,代表性化合物PT10比其他分子表现出更大的量子产率,这与有机溶剂中荧光实验的结果一致。此外,PT10 对 HeLa 细胞中的 SHP1 活性和低细胞毒性显示出选择性荧光反应。最后,根据计算出的优异的双光子吸收特性,表明了未来在双光子细胞荧光成像中的潜在应用。在这项贡献中,我们首先提供了针对SHP1的荧光活化分子PT10,初步实现了SHP1在酶分子水平上的可视化和抑制活性的整合。







































 京公网安备 11010802027423号
京公网安备 11010802027423号