当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity Studies of 3,9-Diazaspiro[5.5]undecane-Based γ-Aminobutyric Acid Type A Receptor Antagonists with Immunomodulatory Effect
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-12-15 , DOI: 10.1021/acs.jmedchem.1c00290
Francesco Bavo 1 , Heleen de-Jong 1 , Jonas Petersen 1, 2 , Christina Birkedahl Falk-Petersen 1 , Rebekka Löffler 1 , Emma Sparrow 3 , Frederik Rostrup 1 , Jannik Nicklas Eliasen 1 , Kristine S Wilhelmsen 1 , Kasper Barslund 4 , Christoffer Bundgaard 4 , Birgitte Nielsen 1 , Uffe Kristiansen 1 , Petrine Wellendorph 1 , Yury Bogdanov 3 , Bente Frølund 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-12-15 , DOI: 10.1021/acs.jmedchem.1c00290
Francesco Bavo 1 , Heleen de-Jong 1 , Jonas Petersen 1, 2 , Christina Birkedahl Falk-Petersen 1 , Rebekka Löffler 1 , Emma Sparrow 3 , Frederik Rostrup 1 , Jannik Nicklas Eliasen 1 , Kristine S Wilhelmsen 1 , Kasper Barslund 4 , Christoffer Bundgaard 4 , Birgitte Nielsen 1 , Uffe Kristiansen 1 , Petrine Wellendorph 1 , Yury Bogdanov 3 , Bente Frølund 1
Affiliation
|
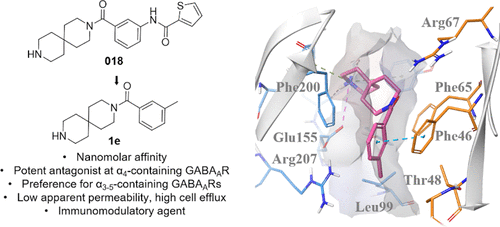
The 3,9-diazaspiro[5.5]undecane-based compounds 2027 and 018 have previously been reported to be potent competitive γ-aminobutyric acid type A receptor (GABAAR) antagonists showing low cellular membrane permeability. Given the emerging peripheral application of GABAAR ligands, we hypothesize 2027 analogs as promising lead structures for peripheral GABAAR inhibition. We herein report a study on the structural determinants of 2027 in order to suggest a potential binding mode as a basis for rational design. The study identified the importance of the spirocyclic benzamide, compensating for the conventional acidic moiety, for GABAAR ligands. The structurally simplified m-methylphenyl analog 1e displayed binding affinity in the high-nanomolar range (Ki = 180 nM) and was superior to 2027 and 018 regarding selectivity for the extrasynaptic α4βδ subtype versus the α1- and α2- containing subtypes. Importantly, 1e was shown to efficiently rescue inhibition of T cell proliferation, providing a platform to explore the immunomodulatory potential for this class of compounds.
中文翻译:

具有免疫调节作用的3,9-二氮杂螺[5.5]十一烷基γ-氨基丁酸A型受体拮抗剂的结构-活性研究
3,9-二氮杂螺[5.5]十一烷基化合物2027和018先前已被报道为有效的竞争性γ-氨基丁酸A型受体(GABA A R)拮抗剂,显示出低细胞膜渗透性。鉴于 GABA A R 配体的新兴外周应用,我们假设2027类似物作为外周 GABA A R 抑制的有前途的先导结构。我们在此报告了一项关于2027 年结构决定因素的研究,以提出一种潜在的结合模式作为合理设计的基础。该研究确定了螺环苯甲酰胺对于 GABA A R 配体的重要性,它可以补偿传统的酸性部分。结构简化的间甲基苯基类似物1e显示出高纳摩尔范围 ( K i = 180 nM) 的结合亲和力,并且在突触外 α 4 βδ 亚型与含 α 1和 α 2亚型的选择性方面优于2027和018亚型。重要的是, 1e被证明可以有效地挽救 T 细胞增殖的抑制,为探索此类化合物的免疫调节潜力提供了一个平台。
更新日期:2021-12-23
中文翻译:

具有免疫调节作用的3,9-二氮杂螺[5.5]十一烷基γ-氨基丁酸A型受体拮抗剂的结构-活性研究
3,9-二氮杂螺[5.5]十一烷基化合物2027和018先前已被报道为有效的竞争性γ-氨基丁酸A型受体(GABA A R)拮抗剂,显示出低细胞膜渗透性。鉴于 GABA A R 配体的新兴外周应用,我们假设2027类似物作为外周 GABA A R 抑制的有前途的先导结构。我们在此报告了一项关于2027 年结构决定因素的研究,以提出一种潜在的结合模式作为合理设计的基础。该研究确定了螺环苯甲酰胺对于 GABA A R 配体的重要性,它可以补偿传统的酸性部分。结构简化的间甲基苯基类似物1e显示出高纳摩尔范围 ( K i = 180 nM) 的结合亲和力,并且在突触外 α 4 βδ 亚型与含 α 1和 α 2亚型的选择性方面优于2027和018亚型。重要的是, 1e被证明可以有效地挽救 T 细胞增殖的抑制,为探索此类化合物的免疫调节潜力提供了一个平台。







































 京公网安备 11010802027423号
京公网安备 11010802027423号