当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic Hydrolysis of Trilactone Siderophores: Where Chiral Recognition Occurs in Enterobactin and Bacillibactin Iron Transport(1)
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-09-09 , DOI: 10.1021/ja903051q Rebecca J Abergel 1 , Anna M Zawadzka , Trisha M Hoette , Kenneth N Raymond
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-09-09 , DOI: 10.1021/ja903051q Rebecca J Abergel 1 , Anna M Zawadzka , Trisha M Hoette , Kenneth N Raymond
Affiliation
|
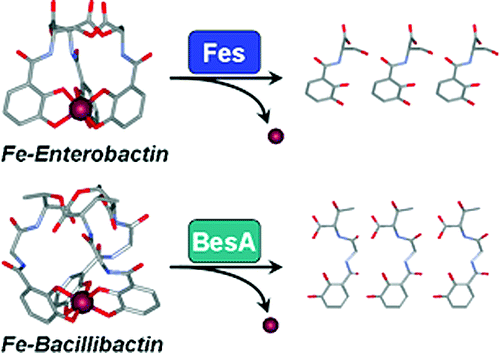
Bacillibactin and enterobactin are hexadentate catecholate siderophores produced by bacteria upon iron limitation to scavenge ferric ion and seem to be the ultimate siderophores of their two respective domains: Gram-positive and Gram-negative. Iron acquisition mediated by these trilactone-based ligands necessitates enzymatic hydrolysis of the scaffold for successful intracellular iron delivery. The esterases BesA and Fes hydrolyze bacillibactin and enterobactin, respectively, as well as the corresponding iron complexes. Bacillibactin binds iron through three 2,3-catecholamide moieties linked to a trithreonine scaffold via glycine spacers, whereas in enterobactin the iron-binding moieties are directly attached to a tri-l-serine backbone; although apparently minor, these structural differences result in markedly different iron coordination properties and iron transport behavior. Comparison of the solution thermodynamic and circular dichroism properties of bacillibactin, enterobactin and the synthetic analogs d-enterobactin, SERGlyCAM and d-SERGlyCAM has determined the role of each different feature in the siderophores' molecular structures in ferric complex stability and metal chirality. While opposite metal chiralities in the different complexes did not affect transport and incorporation in Bacillus subtilis, ferric complexes formed with the various siderophores did not systematically promote growth of the bacteria. The bacillibactin esterase BesA is less specific than the enterobactin esterase Fes; BesA can hydrolyze the trilactones of both siderophores, while only the tri-l-serine trilactone is a substrate of Fes. Both enzymes are stereospecific and cannot cleave tri-d-serine lactones. These data provide a complete picture of the microbial iron transport mediated by these two siderophores, from initial recognition and transport to intracellular iron release.
中文翻译:

三内酯铁载体的酶促水解:在肠杆菌素和杆菌素铁转运中发生手性识别的位置 (1)
Bacillibactin 和 enterobactin 是由细菌在铁限制清除铁离子时产生的六齿儿茶酚酸铁载体,似乎是它们各自两个域的最终铁载体:革兰氏阳性和革兰氏阴性。由这些基于三内酯的配体介导的铁获取需要支架的酶促水解才能成功地在细胞内输送铁。酯酶 BesA 和 Fes 分别水解杆菌素和肠杆菌素,以及相应的铁复合物。杆菌素通过三个 2,3-儿茶酚酰胺部分结合铁,通过甘氨酸间隔物连接到三苏氨酸支架上,而在肠杆菌素中,铁结合部分直接连接到三-l-丝氨酸主链上;虽然看起来很小,这些结构差异导致明显不同的铁配位特性和铁传输行为。比较杆菌素、肠杆菌素和合成类似物 d-肠杆菌素、SERGlyCAM 和 d-SERGlyCAM 的溶液热力学和圆二色性特性,确定了铁载体分子结构中每种不同特征在铁配合物稳定性和金属手性方面的作用。虽然不同复合物中相反的金属手性不影响枯草芽孢杆菌中的运输和掺入,但与各种铁载体形成的铁复合物不会系统地促进细菌的生长。杆菌素酯酶 BesA 的特异性低于肠杆菌素酯酶 Fes;BesA 可以水解两个铁载体的三内酯,而只有三-l-丝氨酸三内酯是 Fes 的底物。这两种酶都是立体特异性的,不能裂解三 d-丝氨酸内酯。这些数据提供了由这两种铁载体介导的微生物铁转运的完整图景,从最初的识别和转运到细胞内铁释放。
更新日期:2009-09-09
中文翻译:

三内酯铁载体的酶促水解:在肠杆菌素和杆菌素铁转运中发生手性识别的位置 (1)
Bacillibactin 和 enterobactin 是由细菌在铁限制清除铁离子时产生的六齿儿茶酚酸铁载体,似乎是它们各自两个域的最终铁载体:革兰氏阳性和革兰氏阴性。由这些基于三内酯的配体介导的铁获取需要支架的酶促水解才能成功地在细胞内输送铁。酯酶 BesA 和 Fes 分别水解杆菌素和肠杆菌素,以及相应的铁复合物。杆菌素通过三个 2,3-儿茶酚酰胺部分结合铁,通过甘氨酸间隔物连接到三苏氨酸支架上,而在肠杆菌素中,铁结合部分直接连接到三-l-丝氨酸主链上;虽然看起来很小,这些结构差异导致明显不同的铁配位特性和铁传输行为。比较杆菌素、肠杆菌素和合成类似物 d-肠杆菌素、SERGlyCAM 和 d-SERGlyCAM 的溶液热力学和圆二色性特性,确定了铁载体分子结构中每种不同特征在铁配合物稳定性和金属手性方面的作用。虽然不同复合物中相反的金属手性不影响枯草芽孢杆菌中的运输和掺入,但与各种铁载体形成的铁复合物不会系统地促进细菌的生长。杆菌素酯酶 BesA 的特异性低于肠杆菌素酯酶 Fes;BesA 可以水解两个铁载体的三内酯,而只有三-l-丝氨酸三内酯是 Fes 的底物。这两种酶都是立体特异性的,不能裂解三 d-丝氨酸内酯。这些数据提供了由这两种铁载体介导的微生物铁转运的完整图景,从最初的识别和转运到细胞内铁释放。















































 京公网安备 11010802027423号
京公网安备 11010802027423号