当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Full Characterization of the UV Hydrodebromination Products of the Current-Use Brominated Flame Retardants Hexabromobenzene, Pentabromotoluene, and Pentabromoethylbenzene
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-12-10 , DOI: 10.1021/acs.est.1c04679
Alexandra Klimm 1 , Walter Vetter 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-12-10 , DOI: 10.1021/acs.est.1c04679
Alexandra Klimm 1 , Walter Vetter 1
Affiliation
|
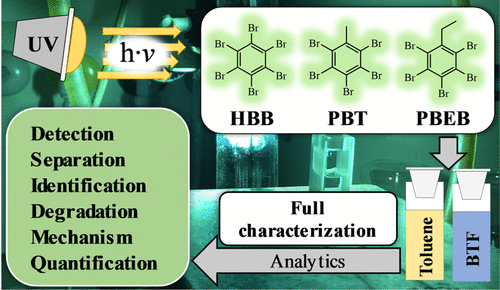
UV transformation was studied with three structurally closely related current-use brominated flame retardants (cuBFRs), i.e., hexabromobenzene (HBB), pentabromotoluene (PBT), and pentabromoethylbenzene (PBEB). Irradiation in toluene and benzotrifluoride (BTF) showed pseudo-first-order kinetics. Repeated high-performance liquid chromatographic (HPLC) fractionation, available reference standards, dedicated syntheses, gas chromatography with mass spectrometry (GC/MS), GC separation on two different phases including retention time rules based on dipole interactions, and proton magnetic resonance spectroscopy (1H NMR) evaluation enabled a full structural characterization of all 22 transformation products formed by hydrodebromination. In addition to pentabromobenzene (only transformation product with five bromine), tetra- and tribrominated transformation products were predominantly formed in the case of all three cuBFRs. Hydrodebromination was favored by bromine removal from positions with a high Br density. Br → H exchange was about 3 times faster in positions flanked by two vicinal Br atoms. This favored pathway explained why hydrodebromination sharply dropped at the level of tribrominated cuBFRs because readily degradable precursors were no more available at this point. Hence, a full degradation of tribrominated and lower-brominated transformation products may only be achieved in combination with a different process such as microbial transformation.
中文翻译:

当前使用的溴化阻燃剂六溴苯、五溴甲苯和五溴乙苯的紫外加氢脱溴产物的全面表征
使用三种结构密切相关的当前使用的溴化阻燃剂 (cuBFRs) 研究了紫外线转化,即六溴苯 (HBB)、五溴甲苯 (PBT) 和五溴乙苯 (PBEB)。甲苯和三氟化苯 (BTF) 中的辐照显示准一级动力学。重复高效液相色谱 (HPLC) 分馏、可用的参考标准、专用合成、气相色谱和质谱 (GC/MS)、两种不同相的 GC 分离,包括基于偶极相互作用的保留时间规则和质子磁共振光谱 ( 1H NMR) 评估能够对通过加氢脱溴形成的所有 22 种转化产物进行完整的结构表征。除了五溴苯(仅含五溴的转化产物)外,在所有三种 cuBFR 的情况下,主要形成四溴化和三溴化转化产物。从高 Br 密度的位置去除溴有利于加氢脱溴。Br → H 交换在两个邻位 Br 原子两侧的位置快约 3 倍。这种有利的途径解释了为什么加氢脱溴在三溴化 cuBFR 水平上急剧下降,因为此时不再有易于降解的前体。因此,三溴化和低溴化转化产物的完全降解可能只有与不同的过程(例如微生物转化)结合才能实现。
更新日期:2021-12-21
中文翻译:

当前使用的溴化阻燃剂六溴苯、五溴甲苯和五溴乙苯的紫外加氢脱溴产物的全面表征
使用三种结构密切相关的当前使用的溴化阻燃剂 (cuBFRs) 研究了紫外线转化,即六溴苯 (HBB)、五溴甲苯 (PBT) 和五溴乙苯 (PBEB)。甲苯和三氟化苯 (BTF) 中的辐照显示准一级动力学。重复高效液相色谱 (HPLC) 分馏、可用的参考标准、专用合成、气相色谱和质谱 (GC/MS)、两种不同相的 GC 分离,包括基于偶极相互作用的保留时间规则和质子磁共振光谱 ( 1H NMR) 评估能够对通过加氢脱溴形成的所有 22 种转化产物进行完整的结构表征。除了五溴苯(仅含五溴的转化产物)外,在所有三种 cuBFR 的情况下,主要形成四溴化和三溴化转化产物。从高 Br 密度的位置去除溴有利于加氢脱溴。Br → H 交换在两个邻位 Br 原子两侧的位置快约 3 倍。这种有利的途径解释了为什么加氢脱溴在三溴化 cuBFR 水平上急剧下降,因为此时不再有易于降解的前体。因此,三溴化和低溴化转化产物的完全降解可能只有与不同的过程(例如微生物转化)结合才能实现。







































 京公网安备 11010802027423号
京公网安备 11010802027423号