当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Insight into the Binding Property and Mechanism of Sulfamethoxazole to Extracellular Proteins of Anammox Sludge
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-12-10 , DOI: 10.1021/acs.est.1c05203 Gui-Feng Li 1 , Wen-Jie Ma 1 , Zhi-Qi Ren 1 , Ye Wang 1 , Jing-Peng Li 1 , Jia-Wen Zhao 1 , Shu-Ting Li 1 , Qi Liu 1 , Ye-Nan Gu 1 , Ya-Fei Cheng 2 , Bao-Cheng Huang 1 , Ren-Cun Jin 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-12-10 , DOI: 10.1021/acs.est.1c05203 Gui-Feng Li 1 , Wen-Jie Ma 1 , Zhi-Qi Ren 1 , Ye Wang 1 , Jing-Peng Li 1 , Jia-Wen Zhao 1 , Shu-Ting Li 1 , Qi Liu 1 , Ye-Nan Gu 1 , Ya-Fei Cheng 2 , Bao-Cheng Huang 1 , Ren-Cun Jin 1
Affiliation
|
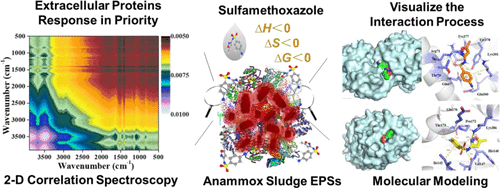
Antibiotics are widely found in nitrogen-containing wastewater, which may affect the operation stability of anaerobic ammonium oxidation (anammox)-based biological treatment systems. Extracellular polymeric substances (EPSs) of anammox sludge play a pivotal role in combining with antibiotics; however, the exact role and how the structure of the leading component of EPSs (i.e., extracellular proteins) changes under antibiotic stress remain to be elucidated. Here, the interaction between sulfamethoxazole and the extracellular proteins of anammox sludge was investigated via multiple spectra and molecular simulation. Results showed that sulfamethoxazole statically quenched the fluorescent components of EPSs, and the quenching constant of the aromatic proteins was the largest, with a value of 1.73 × 104 M–1. The overall binding was an enthalpy-driven process, with ΔH = −75.15 kJ mol–1, ΔS = −0.175 kJ mol–1 K–1, and ΔG = −21.10 kJ mol–1 at 35 °C. The O-P-O and C═O groups responded first under the disturbance of sulfamethoxazole. Excessive sulfamethoxazole (20 mg L–1) would decrease the ratio of α-helix/(β-sheet + random coil) of extracellular proteins, resulting in a loose structure. Molecular docking and dynamic simulation revealed that extracellular proteins would provide abundant sites to bind with sulfamethoxazole, through hydrogen bond and Pi-Akyl hydrophobic interaction forces. Once sulfamethoxazole penetrates into the cell surface and combines with the transmembrane ammonium transport domain, it may inhibit the NH4+ transport. Our findings enhance the understanding on the interaction of extracellular proteins and sulfamethoxazole, which may be valuable for deciphering the response property of anammox sludge under the antibiotic stress.
中文翻译:

磺胺甲恶唑与厌氧氨氧化污泥胞外蛋白结合特性及机制的分子研究
抗生素广泛存在于含氮废水中,可能会影响基于厌氧氨氧化(anammox)的生物处理系统的运行稳定性。厌氧氨氧化污泥的胞外聚合物(EPSs)在与抗生素的结合中起关键作用;然而,在抗生素胁迫下EPS的主要成分(即细胞外蛋白)的确切作用和结构如何变化仍有待阐明。在这里,通过多光谱和分子模拟研究了磺胺甲恶唑与厌氧氨氧化污泥细胞外蛋白之间的相互作用。结果表明,磺胺甲恶唑静态猝灭EPSs的荧光成分,其中芳香族蛋白的猝灭常数最大,为1.73×10 4 M–1。整体结合是一个焓驱动的过程,Δ H = -75.15 kJ mol –1,Δ S = -0.175 kJ mol –1 K –1和 Δ G = -21.10 kJ mol –1在 35 °C。OPO和C=O基团在磺胺甲恶唑的干扰下首先反应。过量的磺胺甲恶唑(20 mg L –1) 会降低细胞外蛋白的 α-螺旋/(β-折叠 + 无规卷曲) 的比例,导致结构松散。分子对接和动态模拟表明,细胞外蛋白将通过氢键和 Pi-Akyl 疏水相互作用力提供丰富的位点与磺胺甲恶唑结合。磺胺甲恶唑一旦渗入细胞表面并与跨膜铵转运结构域结合,就可能抑制NH 4 +转运。我们的研究结果增强了对细胞外蛋白和磺胺甲恶唑相互作用的理解,这可能对破译厌氧氨氧化污泥在抗生素胁迫下的反应特性具有重要意义。
更新日期:2021-12-21
中文翻译:

磺胺甲恶唑与厌氧氨氧化污泥胞外蛋白结合特性及机制的分子研究
抗生素广泛存在于含氮废水中,可能会影响基于厌氧氨氧化(anammox)的生物处理系统的运行稳定性。厌氧氨氧化污泥的胞外聚合物(EPSs)在与抗生素的结合中起关键作用;然而,在抗生素胁迫下EPS的主要成分(即细胞外蛋白)的确切作用和结构如何变化仍有待阐明。在这里,通过多光谱和分子模拟研究了磺胺甲恶唑与厌氧氨氧化污泥细胞外蛋白之间的相互作用。结果表明,磺胺甲恶唑静态猝灭EPSs的荧光成分,其中芳香族蛋白的猝灭常数最大,为1.73×10 4 M–1。整体结合是一个焓驱动的过程,Δ H = -75.15 kJ mol –1,Δ S = -0.175 kJ mol –1 K –1和 Δ G = -21.10 kJ mol –1在 35 °C。OPO和C=O基团在磺胺甲恶唑的干扰下首先反应。过量的磺胺甲恶唑(20 mg L –1) 会降低细胞外蛋白的 α-螺旋/(β-折叠 + 无规卷曲) 的比例,导致结构松散。分子对接和动态模拟表明,细胞外蛋白将通过氢键和 Pi-Akyl 疏水相互作用力提供丰富的位点与磺胺甲恶唑结合。磺胺甲恶唑一旦渗入细胞表面并与跨膜铵转运结构域结合,就可能抑制NH 4 +转运。我们的研究结果增强了对细胞外蛋白和磺胺甲恶唑相互作用的理解,这可能对破译厌氧氨氧化污泥在抗生素胁迫下的反应特性具有重要意义。







































 京公网安备 11010802027423号
京公网安备 11010802027423号