当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Structural Characterization, and Antibacterial and Antifungal Activities of Novel 1,2,4-Triazole Thioether and Thiazolo[3,2-b]-1,2,4-triazole Derivatives Bearing the 6-Fluoroquinazolinyl Moiety
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-12-09 , DOI: 10.1021/acs.jafc.1c02144 Muhan Ding 1 , Suran Wan 1 , Nan Wu 1 , Ya Yan 1 , Junhong Li 1 , Xiaoping Bao 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2021-12-09 , DOI: 10.1021/acs.jafc.1c02144 Muhan Ding 1 , Suran Wan 1 , Nan Wu 1 , Ya Yan 1 , Junhong Li 1 , Xiaoping Bao 1
Affiliation
|
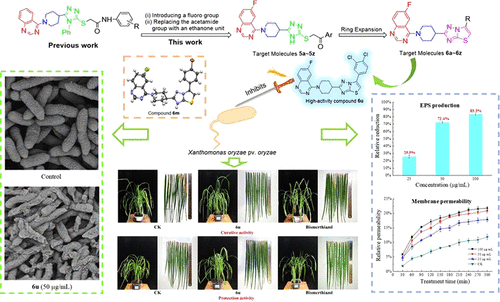
A total of 52 novel 1,2,4-triazole thioether and thiazolo[3,2-b]-1,2,4-triazole derivatives bearing the 6-fluoroquinazolinyl moiety were designed, synthesized, and evaluated as antimicrobial agents in agriculture based on the molecular hybridization strategy. Among them, molecular structures of compounds 5g and 6m were further confirmed via the single-crystal X-ray diffraction method. The bioassay results indicated that some of the target compounds possessed excellent antibacterial activities in vitro against the pathogen Xanthomonas oryzae pv. oryzae (Xoo). For example, compound 6u demonstrated a strong anti-Xoo efficacy with an EC50 value of 18.8 μg/mL, nearly 5-fold more active than that of the commercialized bismerthiazol (EC50 = 93.6 μg/mL). Moreover, the anti-Xoo mechanistic studies revealed that compound 6u exerted its antibacterial effects by increasing the permeability of bacterial membrane, reducing the content of extracellular polysaccharide, and inducing morphological changes of bacterial cells. Importantly, in vivo assays revealed its pronounced protection and curative effects against rice bacterial blight, proving its potential as a promising bactericide candidate for controlling Xoo. Moreover, compound 6u had a good pesticide-likeness based on Tice’s criteria. More interestingly, compound 6u with high anti-Xoo activity also demonstrated a potent inhibitory effect of 80.8% against the fungus Rhizoctonia solani at 50 μg/mL, comparable to that of the commercialized chlorothalonil (85.9%). Overall, the current study will provide useful guidance for the rational design of more efficient agricultural antimicrobial agents using the thiazolo[3,2-b]-1,2,4-triazole derivatives bearing the 6-fluoroquinazolinyl moiety as lead compounds.
中文翻译:

带有 6-氟喹唑啉基部分的新型 1,2,4-三唑硫醚和噻唑并[3,2-b]-1,2,4-三唑衍生物的合成、结构表征以及抗菌和抗真菌活性
共设计、合成了 52 种新型 1,2,4-三唑硫醚和噻唑并[3,2 - b ]-1,2,4-三唑衍生物,它们带有 6-氟喹唑啉基部分,并作为农业抗菌剂进行评估。关于分子杂交策略。其中,化合物5g和6m的分子结构通过单晶X射线衍射法得到进一步证实。生物测定结果表明,部分目标化合物在体外对病原体米黄单胞菌具有优异的抗菌活性。米(Xoo)。例如,化合物6u表现出强大的抗Xoo功效,EC 50值为 18.8 μg/mL,比商业化双甲噻唑的活性高近 5 倍(EC 50 = 93.6 μg/mL)。此外,抗Xoo机理研究表明,化合物6u通过增加细菌膜的通透性,降低细胞外多糖含量,诱导细菌细胞形态变化发挥抗菌作用。重要的是,体内试验揭示了其对水稻白叶枯病的显着保护和治疗效果,证明了其作为控制Xoo的有前景的候选杀菌剂的潜力。此外,根据 Tice 的标准,化合物6u具有良好的农药相似性。更有趣的是,化合物6u具有高抗Xoo活性的50 μg/mL对真菌立枯丝核菌的抑制作用也为 80.8% ,与商业化的百菌清 (85.9%) 相当。总体而言,目前的研究将为使用带有 6-氟喹唑啉基部分的噻唑并[3,2 - b ]-1,2,4-三唑衍生物作为先导化合物合理设计更有效的农业抗菌剂提供有用的指导。
更新日期:2021-12-22
中文翻译:

带有 6-氟喹唑啉基部分的新型 1,2,4-三唑硫醚和噻唑并[3,2-b]-1,2,4-三唑衍生物的合成、结构表征以及抗菌和抗真菌活性
共设计、合成了 52 种新型 1,2,4-三唑硫醚和噻唑并[3,2 - b ]-1,2,4-三唑衍生物,它们带有 6-氟喹唑啉基部分,并作为农业抗菌剂进行评估。关于分子杂交策略。其中,化合物5g和6m的分子结构通过单晶X射线衍射法得到进一步证实。生物测定结果表明,部分目标化合物在体外对病原体米黄单胞菌具有优异的抗菌活性。米(Xoo)。例如,化合物6u表现出强大的抗Xoo功效,EC 50值为 18.8 μg/mL,比商业化双甲噻唑的活性高近 5 倍(EC 50 = 93.6 μg/mL)。此外,抗Xoo机理研究表明,化合物6u通过增加细菌膜的通透性,降低细胞外多糖含量,诱导细菌细胞形态变化发挥抗菌作用。重要的是,体内试验揭示了其对水稻白叶枯病的显着保护和治疗效果,证明了其作为控制Xoo的有前景的候选杀菌剂的潜力。此外,根据 Tice 的标准,化合物6u具有良好的农药相似性。更有趣的是,化合物6u具有高抗Xoo活性的50 μg/mL对真菌立枯丝核菌的抑制作用也为 80.8% ,与商业化的百菌清 (85.9%) 相当。总体而言,目前的研究将为使用带有 6-氟喹唑啉基部分的噻唑并[3,2 - b ]-1,2,4-三唑衍生物作为先导化合物合理设计更有效的农业抗菌剂提供有用的指导。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号