iScience ( IF 4.6 ) Pub Date : 2021-12-09 , DOI: 10.1016/j.isci.2021.103590 Erich J Goebel 1 , Chandramohan Kattamuri 1 , Gregory R Gipson 1 , Lavanya Krishnan 2 , Moises Chavez 2 , Magdalena Czepnik 1 , Michelle C Maguire 2 , Rosa Grenha 2 , Maria Håkansson 3 , Derek T Logan 3 , Asya V Grinberg 4 , Dianne Sako 2 , Roselyne Castonguay 2 , Ravindra Kumar 2 , Thomas B Thompson 1
|
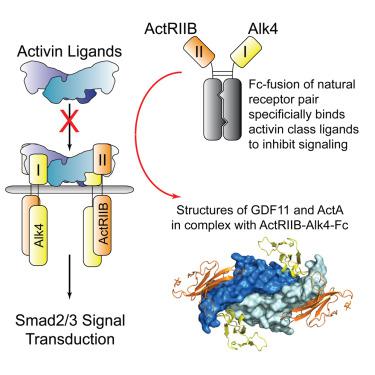
The 30+ unique ligands of the TGFβ family signal by forming complexes using different combinations of type I and type II receptors. Therapeutically, the extracellular domain of a single receptor fused to an Fc molecule can effectively neutralize subsets of ligands. Increased ligand specificity can be accomplished by using the extracellular domains of both the type I and type II receptor to mimic the naturally occurring signaling complex. Here, we report the structure of one “type II-type I-Fc” fusion, ActRIIB-Alk4-Fc, in complex with two TGFβ family ligands, ActA, and GDF11, providing a snapshot of this therapeutic platform. The study reveals that extensive contacts are formed by both receptors, replicating the ternary signaling complex, despite the inherent low affinity of Alk4. Our study shows that low-affinity type I interactions support altered ligand specificity and can be visualized at the molecular level using this platform.
中文翻译:

使用天然 I 型和 II 型 TGFβ 受体组的激活素配体陷阱的结构
TGFβ 家族的 30 多种独特配体通过使用 I 型和 II 型受体的不同组合形成复合物来发出信号。在治疗上,与 Fc 分子融合的单一受体的胞外结构域可以有效地中和配体亚群。通过使用 I 型和 II 型受体的胞外结构域来模拟天然存在的信号复合物,可以提高配体特异性。在这里,我们报告了一种“II 型-I-Fc”融合体 ActRIIB-Alk4-Fc 与两种 TGFβ 家族配体 ActA 和 GDF11 复合物的结构,提供了该治疗平台的概况。研究表明,尽管 Alk4 固有的亲和力较低,但两种受体都形成了广泛的接触,复制了三元信号复合物。我们的研究表明,低亲和力 I 型相互作用支持改变的配体特异性,并且可以使用该平台在分子水平上可视化。































 京公网安备 11010802027423号
京公网安备 11010802027423号