当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isomerization-Induced Excimer Formation of Pyrene-Based Acylhydrazone Controlled by Light- and Solvent-Sensing Aromatic Analytes
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-12-09 , DOI: 10.1021/acs.jpcb.1c07937 Sanjoy Mondal 1 , Aditi Panja 1 , Debabrata Halder 2 , Partha Bairi 1 , Arun K Nandi 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-12-09 , DOI: 10.1021/acs.jpcb.1c07937 Sanjoy Mondal 1 , Aditi Panja 1 , Debabrata Halder 2 , Partha Bairi 1 , Arun K Nandi 1
Affiliation
|
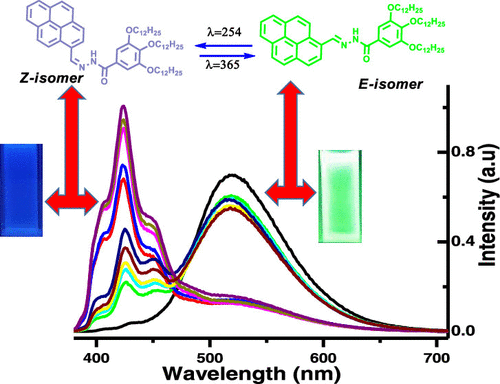
Pyrene is a fluorescent polycyclic aromatic hydrocarbon, and it would be interesting to determine whether its C═N-based conjugate can be used for sensing of aromatic analytes at its supramolecular aggregated state. For this purpose, we have synthesized (E)-3,4,5-tris(dodecyloxy)-N′-(pyren-1-ylmethylene)benzohydrazide (Py@B) by alkylation, substitution, and the Schiff base reaction methodology. The E-isomer of Py@B (E-Py@B) exhibits a bright fluorescence due to excimer formation in nonaromatic solvents. Upon photoirradiation with λ = 254 nm, it exhibits E-Z isomerization across the C═N bond at a low concentration (10–4 M), resulting in a quenched fluorescence intensity, and interestingly, upon photoirradiation with λ = 365 nm, the Z-isomer of Py@B returns to the E-isomer again, indicating that E-Z isomerization of Py@B is reversible in nature. The thick supramolecular aggregated morphology of E-Py@B changes to a flowery needlelike morphology after photoirradiation with λ = 254 nm. The UV–vis absorption band at 370 nm for 10–4 M Py@B in methyl cyclohexane (MCH) is due to excimer formation for closer proximity of pyrene moieties present in E-Py@B and changes to the absorption peak at 344 nm for its Z-isomer formation. The fluorescence spectroscopy results also support the fact that the optimum concentration of the E-isomer of Py@B is 2 × 10–4 M in MCH for excimer formation. From spectral results, it may be concluded that nonaromatic solvents assist in constructing the excimer, but aromatic solvents resist forming an excimer complex of E-Py@B. The fluorescent emission of E-Py@B in MCH is quickly quenched on addition of different aromatic analytes through both static and dynamic pathways. In the solid state, E-Py@B also senses aromatic vapors efficiently via fluorescence quenching. Absorbance spectra of a model molecule obtained using time-dependent density functional theory (TDDFT) calculations on a DFT-optimized structure indicate complex adduct formation between E-Py@B and aromatic analytes from the well-matched theoretical and experimental UV–vis spectra on addition of different analytes with E-Py@B.
中文翻译:

光敏和溶剂敏感芳族分析物控制的芘基酰基腙的异构化诱导准分子形成
芘是一种荧光多环芳烃,确定其 C=N 基共轭物是否可用于检测处于超分子聚集状态的芳烃分析物将是一件有趣的事情。为此,我们通过烷基化、取代和席夫碱反应方法合成了 ( E )-3,4,5-三(十二烷氧基) -N '-(pyren-1-ylmethylene)benzohydrazide (Py@B)。Py@B的E-异构体 ( E -Py@ B) 由于在非芳族溶剂中形成准分子而呈现出明亮的荧光。在 λ = 254 nm 的光照射下,它在低浓度 (10 –4M),导致荧光强度猝灭,有趣的是,在 λ = 365 nm 的光照射下,Py@B 的Z异构体再次返回E异构体,表明Py@B 的EZ异构化本质上是可逆的。E- Py@B的厚超分子聚集形态在 λ = 254 nm 光辐照后变为花针状形态。甲基环己烷 (MCH) 中 10 –4 M Py@B 在370 nm 处的紫外-可见吸收带是由于E -Py@B中存在的芘部分更接近而形成了准分子,并变为 344 nm 处的吸收峰因为它的Z-异构体的形成。荧光光谱结果也支持这样一个事实,即 Py@B 的E异构体在 MCH 中的最佳浓度为 2 × 10 –4 M,用于形成准分子。从光谱结果可以得出结论,非芳族溶剂有助于构建准分子,但芳族溶剂抵抗形成E- Py@B 的准分子复合物。通过静态和动态途径添加不同的芳香族分析物后,MCH 中E -Py@B的荧光发射被快速淬灭。在固态下,E-Py@B 还通过荧光猝灭有效地检测芳香蒸气。在 DFT 优化结构上使用时间相关密度泛函理论 (TDDFT) 计算获得的模型分子的吸光度光谱表明E -Py@B 与芳香族分析物之间形成了复杂的加合物,来自良好匹配的理论和实验 UV-vis 光谱用E -Py@B添加不同的分析物。
更新日期:2021-12-23
中文翻译:

光敏和溶剂敏感芳族分析物控制的芘基酰基腙的异构化诱导准分子形成
芘是一种荧光多环芳烃,确定其 C=N 基共轭物是否可用于检测处于超分子聚集状态的芳烃分析物将是一件有趣的事情。为此,我们通过烷基化、取代和席夫碱反应方法合成了 ( E )-3,4,5-三(十二烷氧基) -N '-(pyren-1-ylmethylene)benzohydrazide (Py@B)。Py@B的E-异构体 ( E -Py@ B) 由于在非芳族溶剂中形成准分子而呈现出明亮的荧光。在 λ = 254 nm 的光照射下,它在低浓度 (10 –4M),导致荧光强度猝灭,有趣的是,在 λ = 365 nm 的光照射下,Py@B 的Z异构体再次返回E异构体,表明Py@B 的EZ异构化本质上是可逆的。E- Py@B的厚超分子聚集形态在 λ = 254 nm 光辐照后变为花针状形态。甲基环己烷 (MCH) 中 10 –4 M Py@B 在370 nm 处的紫外-可见吸收带是由于E -Py@B中存在的芘部分更接近而形成了准分子,并变为 344 nm 处的吸收峰因为它的Z-异构体的形成。荧光光谱结果也支持这样一个事实,即 Py@B 的E异构体在 MCH 中的最佳浓度为 2 × 10 –4 M,用于形成准分子。从光谱结果可以得出结论,非芳族溶剂有助于构建准分子,但芳族溶剂抵抗形成E- Py@B 的准分子复合物。通过静态和动态途径添加不同的芳香族分析物后,MCH 中E -Py@B的荧光发射被快速淬灭。在固态下,E-Py@B 还通过荧光猝灭有效地检测芳香蒸气。在 DFT 优化结构上使用时间相关密度泛函理论 (TDDFT) 计算获得的模型分子的吸光度光谱表明E -Py@B 与芳香族分析物之间形成了复杂的加合物,来自良好匹配的理论和实验 UV-vis 光谱用E -Py@B添加不同的分析物。































 京公网安备 11010802027423号
京公网安备 11010802027423号