Chemosphere ( IF 8.1 ) Pub Date : 2021-12-08 , DOI: 10.1016/j.chemosphere.2021.133198 Shuyu Wang 1 , Zhonglin Chen 1 , Pengwei Yan 1 , Tianhao She 1 , Weiqiang Wang 1 , Lanbo Bi 1 , Jing Kang 1 , Jimin Shen 2 , Xueyan Li 3 , Linlu Shen 1 , Yizhen Cheng 1
|
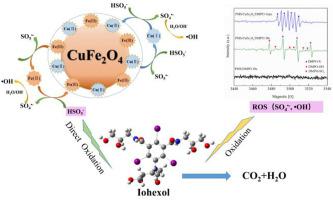
Iohexol as an iodinated X-ray contrast agent is widely used, and it is the potential precursor for toxic iodinated disinfection by-products in the disinfection process. In this study, a series of CuFe2O4 catalysts were prepared by sol-gel method with different molar ratios of total metal cations to citric acid ([Men+]T/CA) and employed as heterogeneous catalysts to activate peroxymonosulfate (PMS) for the removal of iohexol. The catalysts were characterized by various technologies, and the effect of [Men+]T/CA molar ratio on the catalysts' properties was explored. The CuFe2O4 synthesized with [Men+]T/CA molar ratio of 1:1 showed the best catalytic activity to PMS, and 95.0% of 1.0 mg/L iohexol was removed within 15 min by using 50 mg/L CuFe2O4 and 20 mg/L PMS. The quenching experiment and electron spin resonance (ESR) spectra indicated the generation of SO4 - and
- and  OH in the CuFe2O4/PMS system, and the quantity experiments revealed that the generation concentration of SO4
OH in the CuFe2O4/PMS system, and the quantity experiments revealed that the generation concentration of SO4 - was ten times higher than that of
- was ten times higher than that of  OH. The generation mechanism of SO4
OH. The generation mechanism of SO4 - and ·OH were investigated by ATR-FTIR and X-ray photoelectron spectroscopy (XPS) spectra. The effects of catalyst dosage, PMS and iohexol concentration on the removal of iohexol were studied, and various water matrix factors including solution pH, natural organic matter (NOM) concentration and inorganic ions were also considered. Based on the twelve intermediate products of iohexol detected by UPLC-QTOF/MS, the degradation pathway was proposed. The high catalytic activity and reusability of CuFe2O4 indicated that CuFe2O4 activating PMS is an effective and sustainable way for the treatment of iohexol.
- and ·OH were investigated by ATR-FTIR and X-ray photoelectron spectroscopy (XPS) spectra. The effects of catalyst dosage, PMS and iohexol concentration on the removal of iohexol were studied, and various water matrix factors including solution pH, natural organic matter (NOM) concentration and inorganic ions were also considered. Based on the twelve intermediate products of iohexol detected by UPLC-QTOF/MS, the degradation pathway was proposed. The high catalytic activity and reusability of CuFe2O4 indicated that CuFe2O4 activating PMS is an effective and sustainable way for the treatment of iohexol.
中文翻译:

CuFe2O4活化过一硫酸盐增强水中碘海醇的降解:效率、机理和降解途径
碘海醇作为碘化X射线造影剂应用广泛,是消毒过程中有毒碘化消毒副产物的潜在前体。本研究采用溶胶-凝胶法制备了一系列具有不同总金属阳离子与柠檬酸摩尔比([Me n+ ] T /CA)的CuFe 2 O 4催化剂,并将其用作非均相催化剂来活化过氧单硫酸盐(PMS)。用于去除碘海醇。采用多种技术对催化剂进行了表征,探讨了[Me n+ ] T /CA摩尔比对催化剂性能的影响。用[Me n+ ]合成的CuFe 2 O 4T /CA摩尔比为1:1对PMS的催化活性最好,使用50 mg/L CuFe 2 O 4和20 mg/L PMS可在15 min内去除95.0%的1.0 mg/L碘海醇。淬灭实验和电子自旋共振(ESR)光谱表明CuFe 2 O 4 /PMS体系中生成了SO 4  -和
-和 OH,数量实验表明SO 4 -的生成浓度是CuFe 2 O 4 /PMS体系的10倍。哦。SO 4的生成机制——
OH,数量实验表明SO 4 -的生成浓度是CuFe 2 O 4 /PMS体系的10倍。哦。SO 4的生成机制——

 和·OH通过ATR-FTIR和X射线光电子能谱(XPS)光谱进行了研究。研究了催化剂用量、PMS和碘海醇浓度对碘海醇去除率的影响,并考虑了溶液pH、天然有机物(NOM)浓度和无机离子等多种水基质因素。基于UPLC-QTOF/MS检测的12种碘海醇中间产物,提出了降解途径。CuFe 2 O 4的高催化活性和可重复使用性表明CuFe 2 O 4活化PMS是一种有效且可持续的碘海醇处理方法。
和·OH通过ATR-FTIR和X射线光电子能谱(XPS)光谱进行了研究。研究了催化剂用量、PMS和碘海醇浓度对碘海醇去除率的影响,并考虑了溶液pH、天然有机物(NOM)浓度和无机离子等多种水基质因素。基于UPLC-QTOF/MS检测的12种碘海醇中间产物,提出了降解途径。CuFe 2 O 4的高催化活性和可重复使用性表明CuFe 2 O 4活化PMS是一种有效且可持续的碘海醇处理方法。












































 京公网安备 11010802027423号
京公网安备 11010802027423号