Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2021-12-08 , DOI: 10.1016/j.molstruc.2021.132127 Namita A. More 1, 2 , Nitin L. Jadhao 1, 2 , Rohan J. Meshram 3 , Prajakta Tambe 4 , Rajesh A. Salve 4 , Jagjivan K. Sabane 1, 2 , Sanskruti N. Sawant 5 , Virendra Gajbhiye 4 , Jayant M. Gajbhiye 1, 2
|
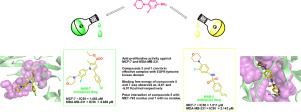
Heterocyclic morpholine compounds are well-known for their anti-cancer activity. In this study, novel morpholine and its sulfonamide derivatives were designed and synthesized as potential anti-tumor agents. The new compounds were obtained from amine derivatives via nucleophilic addition reactions, providing the desired products in 70 to 90% yield. The docking analysis was performed for all thirty-one compounds. Out of them, we represent the docking poses for compounds NAM-5 and NAM-7 as representatives. After docking analysis, compounds NAM-5 and NAM- 7 were tested for in vitro antitumor activity against breast cancer cell lines (MCF-7 and MDA-MB-231) and healthy mouse embryonic fibroblast cell line (3T3L-1). Amongst these, sulfonamide group-containing compound NAM-5 showed significant anti-proliferative activity with IC50 of 1.811 µM and 2.143 µM for MCF-7 and MDA-MB-231 cells, respectively. On the other hand, NAM-7 showed good anti-proliferative activity against MCF-7 (IC50 1.883 µM) but slightly lower activity against MDA-MB-231 cells (IC50 4.688 µM). The activity of both the compound was also tested on 3T3L-1 Cell line which showed activity similar to clinically approved anti-cancer drug doxorubicin (DOX). The cell death analysis by flow-cytometry confirmed apoptosis mediated cell death in 3T3L-1, MCF-7 and MDA-MB-231 cells when treated with the NAM-5 and NAM-7 respectively. The results demonstrated that the synthesized sulfonamide derivatives have significant potential as anti-cancer agents and have a substantial importance in cancer therapeutics with favourable safety profile. Structural analysis of docked poses of sulfonamide derivatives attempts to shed light on the structural basis of sulfonamide derivatives based anti-cancer effect.
中文翻译:

新型 3-fluoro-4-morpholinoaniline 衍生物:乳腺癌细胞抗癌活性的合成和评估
杂环吗啉化合物以其抗癌活性而闻名。在这项研究中,新型吗啉及其磺酰胺衍生物被设计和合成为潜在的抗肿瘤药物。新化合物是通过亲核加成反应从胺衍生物中获得的,以 70% 到 90% 的产率提供所需的产物。对所有 31 种化合物进行对接分析。其中,我们代表化合物 NAM-5 和 NAM-7 的对接姿势作为代表。对接分析后,化合物 NAM-5 和 NAM-7 进行了体外测试针对乳腺癌细胞系(MCF-7 和 MDA-MB-231)和健康小鼠胚胎成纤维细胞系(3T3L-1)的抗肿瘤活性。其中,含有磺酰胺基团的化合物 NAM-5 显示出显着的抗增殖活性,对 MCF-7 和 MDA-MB-231 细胞的IC 50 分别为 1.811 µM 和 2.143 µM。另一方面,NAM -7 对 MCF- 7显示出良好的抗增殖活性(IC 50 1.883 µM),但对 MDA-MB-231 细胞的活性略低(IC 504.688 微米)。还在 3T3L-1 细胞系上测试了这两种化合物的活性,该细胞系显示出与临床批准的抗癌药物多柔比星 (DOX) 相似的活性。通过流式细胞术进行的细胞死亡分析证实,当分别用 NAM-5 和 NAM-7 处理时,3T3L-1、MCF-7 和 MDA-MB-231 细胞中的细胞凋亡介导了细胞死亡。结果表明,合成的磺酰胺衍生物具有作为抗癌剂的巨大潜力,并且在具有良好安全性的癌症治疗中具有重要意义。磺酰胺衍生物对接位姿的结构分析试图阐明基于磺酰胺衍生物的抗癌作用的结构基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号