当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hybridized 4-Trifluoromethyl-(1,2,3-triazol-1-yl)quinoline System: Synthesis, Photophysics, Selective DNA/HSA Bio-interactions and Molecular Docking
ChemBioChem ( IF 2.6 ) Pub Date : 2021-12-08 , DOI: 10.1002/cbic.202100649 Yuri G Kappenberg 1 , Felipe S Stefanello 1 , Nilo Zanatta 1 , Marcos A P Martins 1 , Pablo A Nogara 2 , João B T Rocha 2 , Isadora Tisoco 3 , Bernardo A Iglesias 3 , Helio G Bonacorso 1
ChemBioChem ( IF 2.6 ) Pub Date : 2021-12-08 , DOI: 10.1002/cbic.202100649 Yuri G Kappenberg 1 , Felipe S Stefanello 1 , Nilo Zanatta 1 , Marcos A P Martins 1 , Pablo A Nogara 2 , João B T Rocha 2 , Isadora Tisoco 3 , Bernardo A Iglesias 3 , Helio G Bonacorso 1
Affiliation
|
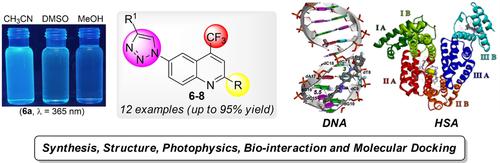
Twelve novel hybridized 4-trifluoromethyl-(1,2,3-triazol-1-yl)quinolines (6–8), were synthesized at 77–95 % yields by a regioselective copper-catalysed azide-alkyne cycloaddition (CuAAC), and characterized by NMR spectroscopy, and single-crystal X-ray diffraction (SC-XRD) analysis. Subsequently, photophysical properties, BSA- and HSA-binding experiments, and molecular docking studies for the new series 6–8 were performed. These are discussed, and compared with similar compounds from recent research.

中文翻译:

杂交 4-三氟甲基-(1,2,3-triazol-1-yl) 喹啉系统:合成、光物理学、选择性 DNA/HSA 生物相互作用和分子对接
通过区域选择性铜催化叠氮化物-炔烃环加成 (CuAAC) 以 77-95% 的产率合成了12种新型杂化 4-三氟甲基-(1,2,3-triazol-1-yl) 喹啉 ( 6-8 ),以及通过NMR光谱和单晶X射线衍射(SC-XRD)分析表征。随后,进行了新系列6-8的光物理特性、BSA 和 HSA 结合实验以及分子对接研究。对这些进行了讨论,并与最近研究中的类似化合物进行了比较。

更新日期:2021-12-08

中文翻译:

杂交 4-三氟甲基-(1,2,3-triazol-1-yl) 喹啉系统:合成、光物理学、选择性 DNA/HSA 生物相互作用和分子对接
通过区域选择性铜催化叠氮化物-炔烃环加成 (CuAAC) 以 77-95% 的产率合成了12种新型杂化 4-三氟甲基-(1,2,3-triazol-1-yl) 喹啉 ( 6-8 ),以及通过NMR光谱和单晶X射线衍射(SC-XRD)分析表征。随后,进行了新系列6-8的光物理特性、BSA 和 HSA 结合实验以及分子对接研究。对这些进行了讨论,并与最近研究中的类似化合物进行了比较。































 京公网安备 11010802027423号
京公网安备 11010802027423号