当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2,2,2-Trifluoroethyl Acetate as an Electrolyte Solvent for Lithium-Ion Batteries: Effect of Weak Solvation on Electrochemical and Structural Characteristics
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.jpcc.1c07312 Saki Sawayama 1 , Riko Ochi 1 , Hideyuki Mimura 2 , Masayuki Morita 1 , Kenta Fujii 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-12-06 , DOI: 10.1021/acs.jpcc.1c07312 Saki Sawayama 1 , Riko Ochi 1 , Hideyuki Mimura 2 , Masayuki Morita 1 , Kenta Fujii 1
Affiliation
|
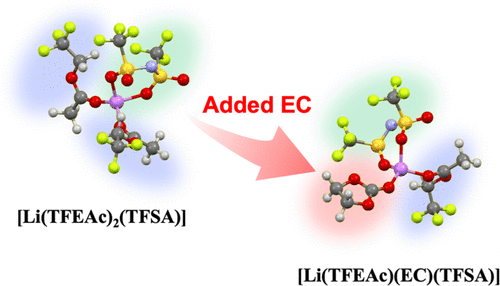
We report the characteristics of 2,2,2-trifluoroethyl acetate (TFEAc) as a new type of electrolyte solvent for lithium (Li)-ion batteries. TFEAc-based electrolyte solutions containing 1.0 mol dm–3 LiTFSA salt [TFSA: bis(trifluoromethanesulfonyl)amide] exhibited a Li-ion insertion reaction into the negative graphite electrode in the first cycle; however, the performance was noticeably degraded during subsequent cycles due to the lack of solid electrolyte interphase (SEI) formation on the electrode. The electrode reaction was markedly improved when a small amount of ethylene carbonate (EC) was added into the LiTFSA/TFEAc solution, which demonstrated a high-rate charge/discharge performance superior to that of the conventional carbonate-based Li-ion battery electrolyte, that is, 1.0 mol dm–3 LiPF6 in the EC + dimethyl carbonate mixture. Quantitative Raman spectral analysis and density functional theory calculations revealed that TFEAc could be categorized as an organic solvent with low solvation power (i.e., with a predicted Gutmann donor number of 9.1); thus, Li ions mainly formed contact ion-pair complexes, [Li(TFEAc)2(TFSA)], in binary LiTFSA/TFEAc solutions. Adding EC into the TFEAc electrolyte modified the Li-ion complex structure; namely, Li ions were coordinated with each TFEAc, EC, and TFSA component to yield [Li(TFEAc)(EC)(TFSA)] as the major species, which coexisted with the ion pair [Li(TFEAc)2(TFSA)]. We discuss the effect of the weak coordination solvent and EC additive on the graphite electrode reaction from the aspects of Li-ion desolvation and SEI formation.
中文翻译:

2,2,2-三氟乙酸乙酯作为锂离子电池的电解质溶剂:弱溶剂化对电化学和结构特性的影响
我们报告了 2,2,2-三氟乙酸乙酯 (TFEAc) 作为一种新型锂 (Li) 离子电池电解质溶剂的特性。含有 1.0 mol dm –3 LiTFSA 盐 [TFSA: 双(三氟甲磺酰基)酰胺]的基于 TFEAc 的电解质溶液在第一次循环中表现出锂离子嵌入石墨负极的反应;然而,由于电极上没有形成固体电解质中间相 (SEI),因此在随后的循环中性能明显下降。当在 LiTFSA/TFEAc 溶液中加入少量碳酸亚乙酯 (EC) 时,电极反应显着改善,表现出优于传统碳酸酯基锂离子电池电解液的高倍率充放电性能,即 1.0 mol dm –3EC+碳酸二甲酯混合物中的LiPF 6。定量拉曼光谱分析和密度泛函理论计算表明,TFEAc 可归类为溶剂化能力低的有机溶剂(即预测的古特曼供体数为 9.1);因此,锂离子主要在二元 LiTFSA/TFEAc 溶液中形成接触离子对复合物 [Li(TFEAc) 2 (TFSA)]。在TFEAc电解液中加入EC改变了锂离子络合物结构;即,锂离子与每个 TFEAc、EC 和 TFSA 组分配位以产生 [Li(TFEAc)(EC)(TFSA)] 作为主要物质,其与离子对 [Li(TFEAc) 2(TFSA)]。我们从锂离子去溶剂化和 SEI 形成方面讨论了弱配位溶剂和 EC 添加剂对石墨电极反应的影响。
更新日期:2021-12-16
中文翻译:

2,2,2-三氟乙酸乙酯作为锂离子电池的电解质溶剂:弱溶剂化对电化学和结构特性的影响
我们报告了 2,2,2-三氟乙酸乙酯 (TFEAc) 作为一种新型锂 (Li) 离子电池电解质溶剂的特性。含有 1.0 mol dm –3 LiTFSA 盐 [TFSA: 双(三氟甲磺酰基)酰胺]的基于 TFEAc 的电解质溶液在第一次循环中表现出锂离子嵌入石墨负极的反应;然而,由于电极上没有形成固体电解质中间相 (SEI),因此在随后的循环中性能明显下降。当在 LiTFSA/TFEAc 溶液中加入少量碳酸亚乙酯 (EC) 时,电极反应显着改善,表现出优于传统碳酸酯基锂离子电池电解液的高倍率充放电性能,即 1.0 mol dm –3EC+碳酸二甲酯混合物中的LiPF 6。定量拉曼光谱分析和密度泛函理论计算表明,TFEAc 可归类为溶剂化能力低的有机溶剂(即预测的古特曼供体数为 9.1);因此,锂离子主要在二元 LiTFSA/TFEAc 溶液中形成接触离子对复合物 [Li(TFEAc) 2 (TFSA)]。在TFEAc电解液中加入EC改变了锂离子络合物结构;即,锂离子与每个 TFEAc、EC 和 TFSA 组分配位以产生 [Li(TFEAc)(EC)(TFSA)] 作为主要物质,其与离子对 [Li(TFEAc) 2(TFSA)]。我们从锂离子去溶剂化和 SEI 形成方面讨论了弱配位溶剂和 EC 添加剂对石墨电极反应的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号