当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allylic C−H Amination for the Preparation ofsyn-1,3-Amino Alcohol Motifs
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-08-26 , DOI: 10.1021/ja9054959 Grant T Rice 1 , M Christina White
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-08-26 , DOI: 10.1021/ja9054959 Grant T Rice 1 , M Christina White
Affiliation
|
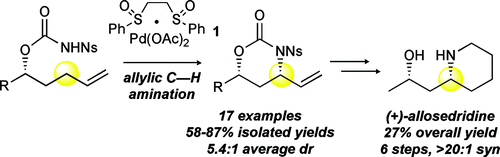
A highly selective and general Pd/sulfoxide-catalyzed allylic C-H amination reaction en route to syn-1,3-amino alcohol motifs is reported. Key to achieving this reactivity under mild conditions is the use of electron-deficient N-nosyl carbamate nucleophiles that are thought to promote functionalization by furnishing higher concentrations of anionic species in situ. The reaction is shown to be orthogonal to classical C-C bond-forming/-reduction sequences as well as nitrene-based C-H amination methods.
中文翻译:

用于制备syn-1,3-氨基醇基序的烯丙C-H 胺化
报告了一种高选择性和通用的 Pd/亚砜催化的烯丙基 CH 胺化反应,用于合成 1,3-氨基醇基序。在温和条件下实现这种反应性的关键是使用缺电子的 N-nosyl 氨基甲酸酯亲核试剂,这些亲核试剂被认为通过原位提供更高浓度的阴离子物质来促进功能化。该反应显示出与经典的 CC 键形成/还原序列以及基于氮烯的 CH 胺化方法正交。
更新日期:2009-08-26
中文翻译:

用于制备syn-1,3-氨基醇基序的烯丙C-H 胺化
报告了一种高选择性和通用的 Pd/亚砜催化的烯丙基 CH 胺化反应,用于合成 1,3-氨基醇基序。在温和条件下实现这种反应性的关键是使用缺电子的 N-nosyl 氨基甲酸酯亲核试剂,这些亲核试剂被认为通过原位提供更高浓度的阴离子物质来促进功能化。该反应显示出与经典的 CC 键形成/还原序列以及基于氮烯的 CH 胺化方法正交。































 京公网安备 11010802027423号
京公网安备 11010802027423号