Fuel ( IF 6.7 ) Pub Date : 2021-12-04 , DOI: 10.1016/j.fuel.2021.122747
Yongzhao Zhang 1, 2 , Yifan Li 1, 2 , Zequan Zeng 1 , Jiangliang Hu 3 , Zhanggen Huang 1, 2, 4
|
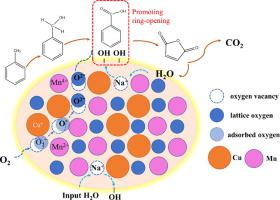
To elucidate the mechanism of the promotion effect of CuMn2O4 catalyst modification with NaOH on toluene oxidation, NaOH-modified CuMn2O4 catalysts with similar physical structures and chemical properties were fabricated through a mild impregnation strategy. The results showed that the NaOH species had a remarkable promotion effect on the CuMn2O4 catalytic performance without lattice oxygen activity improvement. The 2NaOH/CuMn2O4 showed the best catalytic activity with the T90 (the temperature corresponding to 90% conversion) of 188 °C, which was 20 °C lower than that of the unmodified CuMn2O4. In situ diffuse reflectance infrared Fourier-transform spectroscopy (DRIFT)S study revealed that carbonate and benzoate were the main intermediates over CuMn2O4, and the NaOH introduction inhibited carbonate formation and accelerated the degradation of the benzoate aromatic ring. The H2O present in the reaction system could be dissociated into hydroxyl groups under the action of NaOH to form a hydroxyl-rich environment, which boosted the ring-opening of benzoate. In addition, the toluene adsorption experiments confirmed that the nucleophilic lattice oxygen species broke the activated aromatic ring, and the toluene oxidation followed the Mars–van Krevelen mechanism. In this study, the mechanism of the promotion effect of catalyst modification with NaOH on toluene oxidation was revealed, which is of great significance for the design of high-efficiency toluene degradation catalysts.
中文翻译:

CuMn2O4 NaOH 改性对甲苯氧化的促进机制:促进苯甲酸盐的开环
为了阐明的的CuMn的促进作用的机制2 ö 4,用NaOH对甲苯氧化催化剂改性,氢氧化钠改性的CuMn 2 ö 4种催化剂具有相似的物理结构和化学性质进行了通过温和浸渍策略制造。结果表明,NaOH物种对CuMn 2 O 4催化性能有显着的促进作用,而晶格氧活性没有提高。2NaOH/CuMn 2 O 4表现出最好的催化活性,T 90(转化率90%对应的温度)为188°C,比未改性的CuMn 2低20°C○ 4。原位漫反射红外傅里叶变换光谱 (DRIFT) S 研究表明,碳酸盐和苯甲酸盐是 CuMn 2 O 4的主要中间体,NaOH 的引入抑制了碳酸盐的形成并加速了苯甲酸盐芳环的降解。反应体系中存在的H 2 O在NaOH的作用下可解离成羟基,形成富含羟基的环境,促进苯甲酸盐的开环。此外,甲苯吸附实验证实亲核晶格氧物种破坏了活化的芳环,甲苯氧化跟随Mars-van 克雷维伦机制。本研究揭示了NaOH改性催化剂对甲苯氧化的促进作用机理,对高效甲苯降解催化剂的设计具有重要意义。







































 京公网安备 11010802027423号
京公网安备 11010802027423号