当前位置:
X-MOL 学术
›
ACS Appl. Polym. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocured Cationic Polyoxazoline Macromonomers as Gel Polymer Electrolytes for Lithium-Ion Batteries
ACS Applied Polymer Materials ( IF 4.4 ) Pub Date : 2021-12-03 , DOI: 10.1021/acsapm.1c01171 Mathias Drews 1 , Tobias Trötschler 2, 3 , Manuel Bauer 1 , Apurupa Guntupalli 1 , Witali Beichel 1 , Harald Gentischer 1 , Rolf Mülhaupt 2, 3 , Benjamin Kerscher 2 , Daniel Biro 1
ACS Applied Polymer Materials ( IF 4.4 ) Pub Date : 2021-12-03 , DOI: 10.1021/acsapm.1c01171 Mathias Drews 1 , Tobias Trötschler 2, 3 , Manuel Bauer 1 , Apurupa Guntupalli 1 , Witali Beichel 1 , Harald Gentischer 1 , Rolf Mülhaupt 2, 3 , Benjamin Kerscher 2 , Daniel Biro 1
Affiliation
|
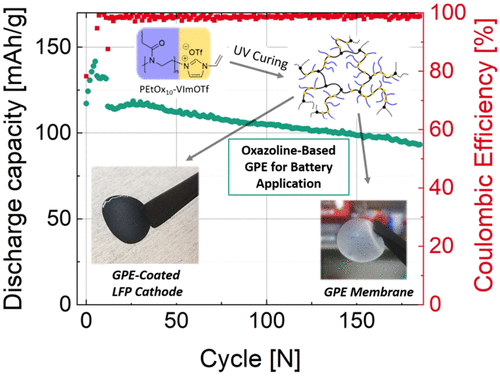
In this work, we present a cationic vinylimidazolium-terminated poly(2-ethyl-2-oxazoline) (PEtOx) macromonomer as a key component of gel polymer electrolytes (GPE) for lithium-ion batteries. GPE production followed a scalable process based on UV curing of the cationic PEtOx macromonomer with polyfunctional acrylic comonomers dissolved in an organic electrolyte (LP30), affording electrolyte-swollen polymeric ionic liquid (PIL) networks with PEtOx side chains. Thus, cathodes coated with a GPE layer of less than 200 μm thickness were readily manufactured. The PIL brush-type GPE is highly insoluble but swellable in LP30 and exhibits pronounced electrolyte retaining ability against evaporation. At 160 °C, the weight loss of the GPE amounted to around 5%. This is 12% less compared to an LP30-soaked commercial Celgard separator. At room temperature, the ionic conductivity was 3.6 × 10–4 S/cm, surpassing that of a comparable Celgard/LP30 system. Contrary to LP30 in Celgard, conductivity measurements for the PIL brush GPE did not indicate any crystallization of the liquid electrolyte at subambient temperatures. This was confirmed by differential scanning calorimetry, suggesting improved ionic mobility in the GPE over a wide temperature range. The electrochemical stability window of the PIL brush GPE is wide enough and fits all common lithium-ion cathode materials. In fact, the GPE exhibited exceptional oxidative stability of 5.2 V vs Li/Li+. Half-cell cycling experiments using a lithium iron phosphate cathode revealed high capacity values of 150 mAh/g at a current rate of C/10. When the current was increased to C/2, the capacity decreased to 120 mAh/g and the cell reached 80% of its initial capacity (referred to C/2) after 180 cycles. Thus, according to the first physicochemical and electrochemical investigations, the PEtOx-based PIL brush GPE represents a promising candidate with respect to lithium-ion battery operation.
中文翻译:

光固化阳离子聚恶唑啉大单体作为锂离子电池的凝胶聚合物电解质
在这项工作中,我们提出了一种阳离子乙烯基咪唑鎓封端的聚 (2-乙基-2-恶唑啉) (PEtOx) 大单体作为锂离子电池凝胶聚合物电解质 (GPE) 的关键成分。GPE 生产遵循可扩展的工艺,该工艺基于阳离子 PEtOx 大分子单体与溶解在有机电解质 (LP30) 中的多官能丙烯酸共聚单体的 UV 固化,从而提供具有 PEtOx 侧链的电解质溶胀聚合物离子液体 (PIL) 网络。因此,涂有厚度小于 200 μm 的 GPE 层的阴极很容易制造。PIL 刷型 GPE 在 LP30 中高度不溶但可溶胀,并表现出显着的电解液保持能力以防止蒸发。在 160 °C 时,GPE 的重量损失约为 5%。与 LP30 浸泡的商用 Celgard 分离器相比,这减少了 12%。在室温下,–4 S/cm,超过同类 Celgard/LP30 系统。与 Celgard 中的 LP30 不同,PIL 刷 GPE 的电导率测量并未表明液体电解质在低于环境温度下有任何结晶。差示扫描量热法证实了这一点,表明 GPE 在很宽的温度范围内离子迁移率有所提高。PIL刷GPE的电化学稳定性窗口足够宽,适合所有常见的锂离子正极材料。事实上,GPE 表现出 5.2 V vs Li/Li +的出色氧化稳定性. 使用磷酸铁锂阴极的半电池循环实验显示,在 C/10 的电流速率下具有 150 mAh/g 的高容量值。当电流增加到 C/2 时,容量下降到 120 mAh/g,循环 180 次后电池达到其初始容量的 80%(称为 C/2)。因此,根据第一次物理化学和电化学研究,基于 PEtOx 的 PIL 刷 GPE 代表了锂离子电池操作的有希望的候选者。
更新日期:2022-01-14
中文翻译:

光固化阳离子聚恶唑啉大单体作为锂离子电池的凝胶聚合物电解质
在这项工作中,我们提出了一种阳离子乙烯基咪唑鎓封端的聚 (2-乙基-2-恶唑啉) (PEtOx) 大单体作为锂离子电池凝胶聚合物电解质 (GPE) 的关键成分。GPE 生产遵循可扩展的工艺,该工艺基于阳离子 PEtOx 大分子单体与溶解在有机电解质 (LP30) 中的多官能丙烯酸共聚单体的 UV 固化,从而提供具有 PEtOx 侧链的电解质溶胀聚合物离子液体 (PIL) 网络。因此,涂有厚度小于 200 μm 的 GPE 层的阴极很容易制造。PIL 刷型 GPE 在 LP30 中高度不溶但可溶胀,并表现出显着的电解液保持能力以防止蒸发。在 160 °C 时,GPE 的重量损失约为 5%。与 LP30 浸泡的商用 Celgard 分离器相比,这减少了 12%。在室温下,–4 S/cm,超过同类 Celgard/LP30 系统。与 Celgard 中的 LP30 不同,PIL 刷 GPE 的电导率测量并未表明液体电解质在低于环境温度下有任何结晶。差示扫描量热法证实了这一点,表明 GPE 在很宽的温度范围内离子迁移率有所提高。PIL刷GPE的电化学稳定性窗口足够宽,适合所有常见的锂离子正极材料。事实上,GPE 表现出 5.2 V vs Li/Li +的出色氧化稳定性. 使用磷酸铁锂阴极的半电池循环实验显示,在 C/10 的电流速率下具有 150 mAh/g 的高容量值。当电流增加到 C/2 时,容量下降到 120 mAh/g,循环 180 次后电池达到其初始容量的 80%(称为 C/2)。因此,根据第一次物理化学和电化学研究,基于 PEtOx 的 PIL 刷 GPE 代表了锂离子电池操作的有希望的候选者。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号