Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Platinum Crosslinked Carbon Dot@TiO2−x p-n Junctions for Relapse-Free Sonodynamic Tumor Eradication via High-Yield ROS and GSH Depletion
Small ( IF 13.0 ) Pub Date : 2021-12-02 , DOI: 10.1002/smll.202103528 Bijiang Geng 1 , Shuang Xu 1 , Ping Li 2 , Xiaokai Li 3 , Fuling Fang 1 , Dengyu Pan 1 , Longxiang Shen 4
Small ( IF 13.0 ) Pub Date : 2021-12-02 , DOI: 10.1002/smll.202103528 Bijiang Geng 1 , Shuang Xu 1 , Ping Li 2 , Xiaokai Li 3 , Fuling Fang 1 , Dengyu Pan 1 , Longxiang Shen 4
Affiliation
|
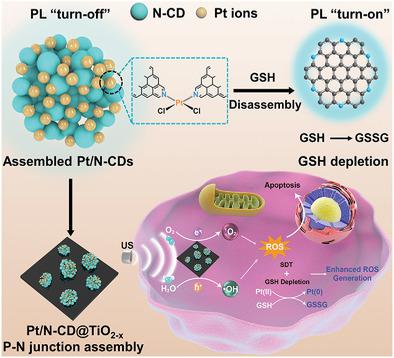
Sonodynamic therapy as a promising noninvasive modality is being developed for tumor therapy, but there is a lack of next-generation sonosensitizers that can generate full ROS at high yields and simultaneously deplete elevated levels of glutathione (GSH) in tumor cells. Semiconductor p-n junctions are engineered as high-efficacy sonosensitizers for sonodynamic tumor eradication using pyridine N-doped carbon dots (N-CDs) as a p-type semiconductor and oxygen-deficient TiO2−x nanosheets as a n-type semiconductor. The rate constants of 1O2 and •OH generation by ultrasound-excited N-CD@TiO2−x p-n junctions are 4.3 and 4.5 times higher than those of TiO2, respectively. A Z-scheme carrier migration mechanism in the p-n junction achieving the rapid spatial separation of the ultrasound-generated electron–hole pairs for enhanced full ROS production is proposed. GSH-cleavable, Pt-crosslinked, N-doped CD fluorescent probes to detect the presence of intracellular GSH are also constructed. A GSH-responsive, p-n junction platform (Pt/N-CD@TiO2−x) with integrated GSH detection, GSH depletion, and enhanced sonodynamic performance is then assembled. Malignant tumors are completely eradicated without relapse via intravenous administration of low-dose Pt/N-CD@TiO2−x under ultrasound irradiation. This work substantiates the great potential of biocompatible, GSH-responsive p-n junctions as next-generation sonosensitizers via p-n junction-enhanced ROS generation and metal ion oxidation of intracellular GSH.
中文翻译:

铂交联碳点@TiO2−x pn 结通过高产 ROS 和 GSH 消耗实现无复发声动力肿瘤根除
声动力疗法作为一种很有前途的非侵入性方式正在被开发用于肿瘤治疗,但缺乏能够以高产率产生完全 ROS 并同时消耗肿瘤细胞中升高的谷胱甘肽 (GSH) 水平的下一代声敏剂。半导体 pn 结被设计为高效声敏剂,用于使用吡啶 N 掺杂碳点 (N-CD) 作为p型半导体和缺氧 TiO 2- x纳米片作为n型半导体来根除声动力学肿瘤。超声激发 N-CD@TiO 2- x pn 结产生1 O 2和 •OH的速率常数是TiO的 4.3 和 4.5 倍2,分别。提出了一种在 pn 结中实现 Z 型载流子迁移机制,实现超声产生的电子-空穴对的快速空间分离,以增强全 ROS 的产生。还构建了用于检测细胞内 GSH 存在的 GSH 可切割、Pt 交联、N 掺杂的 CD 荧光探针。然后组装了一个 GSH 响应的 pn 结平台 (Pt/N-CD@TiO 2− x ),该平台具有集成的 GSH 检测、GSH 消耗和增强的声动力学性能。静脉注射低剂量Pt/N-CD@TiO 2− x可彻底根除恶性肿瘤且不复发在超声波照射下。这项工作通过 pn 结增强 ROS 生成和细胞内 GSH 的金属离子氧化,证实了生物相容性、GSH 响应性 pn 结作为下一代声敏剂的巨大潜力。
更新日期:2022-02-10
中文翻译:

铂交联碳点@TiO2−x pn 结通过高产 ROS 和 GSH 消耗实现无复发声动力肿瘤根除
声动力疗法作为一种很有前途的非侵入性方式正在被开发用于肿瘤治疗,但缺乏能够以高产率产生完全 ROS 并同时消耗肿瘤细胞中升高的谷胱甘肽 (GSH) 水平的下一代声敏剂。半导体 pn 结被设计为高效声敏剂,用于使用吡啶 N 掺杂碳点 (N-CD) 作为p型半导体和缺氧 TiO 2- x纳米片作为n型半导体来根除声动力学肿瘤。超声激发 N-CD@TiO 2- x pn 结产生1 O 2和 •OH的速率常数是TiO的 4.3 和 4.5 倍2,分别。提出了一种在 pn 结中实现 Z 型载流子迁移机制,实现超声产生的电子-空穴对的快速空间分离,以增强全 ROS 的产生。还构建了用于检测细胞内 GSH 存在的 GSH 可切割、Pt 交联、N 掺杂的 CD 荧光探针。然后组装了一个 GSH 响应的 pn 结平台 (Pt/N-CD@TiO 2− x ),该平台具有集成的 GSH 检测、GSH 消耗和增强的声动力学性能。静脉注射低剂量Pt/N-CD@TiO 2− x可彻底根除恶性肿瘤且不复发在超声波照射下。这项工作通过 pn 结增强 ROS 生成和细胞内 GSH 的金属离子氧化,证实了生物相容性、GSH 响应性 pn 结作为下一代声敏剂的巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号